257192
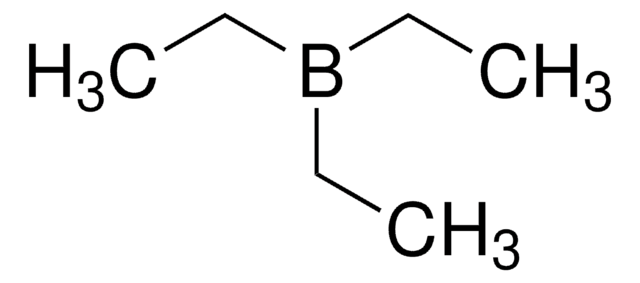
Triethylborane
≥95%
Sinónimos:
Triethylboron
About This Item
Productos recomendados
assay
≥95%
reaction suitability
reagent type: reductant
refractive index
n20/D 1.397 (lit.)
bp
95 °C (lit.)
mp
−93 °C (lit.)
density
0.677 g/mL at 25 °C (lit.)
SMILES string
CCB(CC)CC
InChI
1S/C6H15B/c1-4-7(5-2)6-3/h4-6H2,1-3H3
InChI key
LALRXNPLTWZJIJ-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
- Enantioselective umpolung allylation of aldehydes
- Preparation of tetramethylammonium trialkylphenylborate salts
Catalyst for:
- Radical reductions of alkyl bromides and iodides bearing electron withdrawing groups with N-heterocyclic carbene boranes
- Synthesis of 1-substituted pyrrolines by N-diallylation of amines and ring-closing metathesis
- Decarboxylative C-C bond cleavage reactions
- Alkene hydrogenations
- Aminyl radical cyclizations onto silyl enol ethers
Modifier for single-site organochromium ethylene polymerization catalysts
Packaging
Compatible with the following:
- Aldrich® Sure/Pac™ station for liquefied gases Z566446
- PTFE Sealing tape Z104388 or Z221880
- Straight septum-inlet adapter Z118141 with septa Z565687 or Z565695
Legal Information
Optional
Septum inlet adapter
also commonly purchased with this product
signalword
Danger
hcodes
Hazard Classifications
Acute Tox. 3 Oral - Eye Dam. 1 - Pyr. Liq. 1 - Skin Corr. 1A
Storage Class
4.2 - Pyrophoric and self-heating hazardous materials
wgk_germany
WGK 1
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
Certificados de análisis (COA)
¿No ve la versión correcta?
Si necesita una versión concreta, puede buscar un certificado específico por el número de lote.
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Artículos
We carry a large variety of electrophiles and nucleophiles that are widely used in C–C bond-forming reactions. This group of products contains many organometallic reagents as well as commonly-used alkylating and acylating reagents.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico