195030
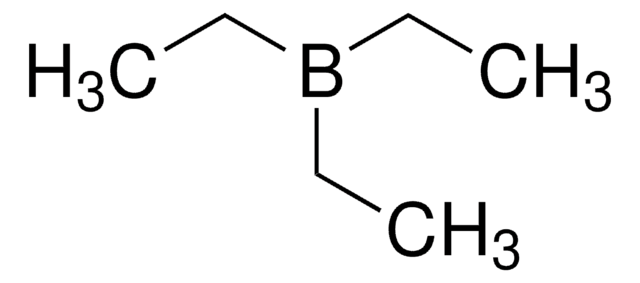
Triethylborane solution
1.0 M in hexanes
Sinónimos:
Triethylboron
About This Item
Productos recomendados
form
liquid
Quality Level
reaction suitability
reagent type: reductant
concentration
1.0 M in hexanes
density
0.675 g/mL at 25 °C
SMILES string
CCB(CC)CC
InChI
1S/C6H15B/c1-4-7(5-2)6-3/h4-6H2,1-3H3
InChI key
LALRXNPLTWZJIJ-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Application
- Allylation of aldehydes
- Decarboxylative C-C bond cleavage reactions
- Rhenium hydride / boron Lewis acid cocatalysis of alkene hydrogenations
- Regioselective hydroxyalkylation of unsaturated oxime ethers
Reactant for radical reductions of alkyl bromides with N-heterocyclic carbene boranes
Reactant for synthesis of tetramethylammonium trialkylphenylborate salts with oxidation potential
- As a radical initiator and terminator of free-radical reactions in aqueous media.(1)
- To synthesize polymers such as poly(2-substituted-1-propenylene)s by reacting with 2-substituted allylic arsonium ylides.(2)
signalword
Danger
Hazard Classifications
Aquatic Chronic 2 - Asp. Tox. 1 - Eye Dam. 1 - Flam. Liq. 2 - Repr. 2 - Skin Corr. 1A - STOT RE 1 Inhalation - STOT SE 3
target_organs
Central nervous system, Nervous system
Storage Class
4.3 - Hazardous materials which set free flammable gases upon contact with water
wgk_germany
WGK 3
flash_point_f
-32.8 °F
flash_point_c
-36 °C
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico