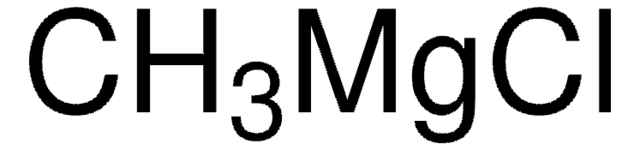254363
Methylmagnesium iodide solution
3.0 M in diethyl ether
Sinónimos:
Iodomethylmagnesium, Methylmagnesium iodine
About This Item
Productos recomendados
Quality Level
reaction suitability
reaction type: Grignard Reaction
concentration
3.0 M in diethyl ether
density
1.261 g/mL at 25 °C
SMILES string
C[Mg]I
InChI
1S/CH3.HI.Mg/h1H3;1H;/q;;+1/p-1
InChI key
AUPXBVDHVRZMIB-UHFFFAOYSA-M
¿Está buscando productos similares? Visita Guía de comparación de productos
Application
- To prepare cis-1,2-dialkylcyclopropanols by treating with titanium(IV) alkoxide and carboxylic esters.
- In stereospecific Ni catalyzed Kumada cross-coupling reactions of benzylic ethers.
- To prepare 2,4-disubstituted selenochromenes by reacting with 1-benzoselenopyrylium salts.
- In one of the key synthetic steps for the preparation of 7β-methyl-substituted 5-androstene derivatives from 3β-acetoxyandrost-5-en-17-one stereoselectively.
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 1 - Skin Corr. 1B - STOT SE 3 - Water-react 1
target_organs
Central nervous system
supp_hazards
Storage Class
4.3 - Hazardous materials which set free flammable gases upon contact with water
wgk_germany
WGK 3
flash_point_f
-40.0 °F - closed cup
flash_point_c
-40 °C - closed cup
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
We carry a large variety of electrophiles and nucleophiles that are widely used in C–C bond-forming reactions. This group of products contains many organometallic reagents as well as commonly-used alkylating and acylating reagents.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico