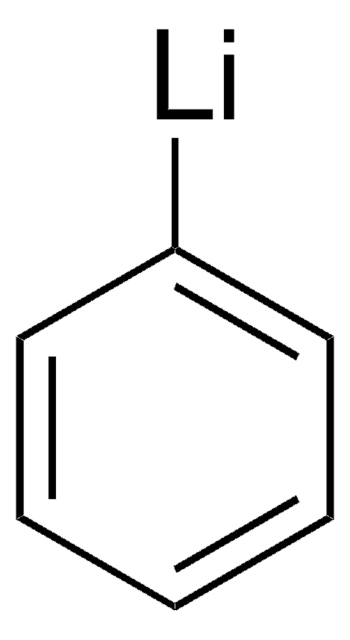
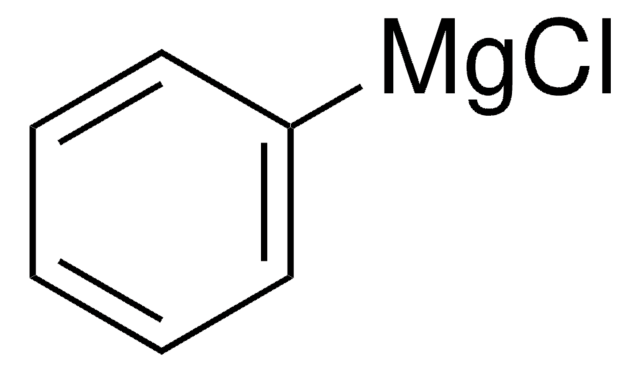
171565
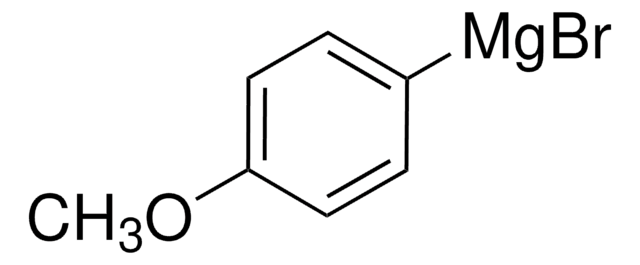
Phenylmagnesium bromide solution
3.0 M in diethyl ether
Sinónimos:
Bromomagnesiobenzene, Bromophenylmagnesium
About This Item
Productos recomendados
Quality Level
reaction suitability
reaction type: Grignard Reaction
concentration
3.0 M in diethyl ether
density
1.134 g/mL at 25 °C
SMILES string
Br[Mg]c1ccccc1
InChI
1S/C6H5.BrH.Mg/c1-2-4-6-5-3-1;;/h1-5H;1H;/q;;+1/p-1
InChI key
ANRQGKOBLBYXFM-UHFFFAOYSA-M
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
General description
Application
It may be used for synthesis of the following:
- 1,3,3-trimethyl-6-phenyl-2-oxabicyclo[2.2.2]octan-6-ol
- 6-benzyl-1,3,3-trimethyl-2-oxabicyclo[2.2.2]octan-6-ol
- (3-(2-Dithiobenzoatepropionyl)propyl)dimethylmethoxysilane, reversible addition-fragmentation chain transfer polymerization (RAFT)-silane agent
- series of o-substituted benzophenones
Packaging
Other Notes
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Flam. Liq. 2 - Skin Corr. 1B - STOT SE 3
target_organs
Respiratory system
supp_hazards
Storage Class
3 - Flammable liquids
wgk_germany
WGK 3
flash_point_f
-40.0 °F - closed cup
flash_point_c
-40 °C - closed cup
ppe
Faceshields, Gloves, Goggles, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
We carry a large variety of electrophiles and nucleophiles that are widely used in C–C bond-forming reactions. This group of products contains many organometallic reagents as well as commonly-used alkylating and acylating reagents.
Global Trade Item Number
| Número de referencia del producto (SKU) | GTIN |
|---|---|
| S892858-1EA | |
| 171565-18L | 4061838751065 |
| 171565-50ML | 4061838751072 |
| 171565-800ML | 4061838751089 |
| 171565-100ML | 4061838751058 |
| 171565-4X25ML | 4065268444450 |
| 171565-8L |
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico