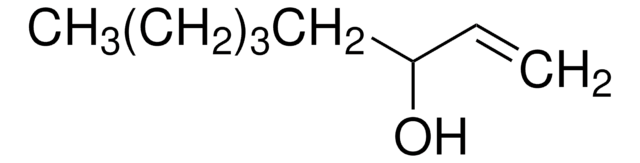W280523
1-Octen-3-ol
natural, ≥95%, FG
Synonym(s):
Pentyl vinyl carbinol
About This Item
Recommended Products
grade
FG
Fragrance grade
Halal
Kosher
natural
Quality Level
Agency
follows IFRA guidelines
reg. compliance
EU Regulation 1223/2009
EU Regulation 1334/2008 & 178/2002
FDA 21 CFR 172.515
Assay
≥95%
greener alternative product characteristics
Less Hazardous Chemical Syntheses
Use of Renewable Feedstocks
Learn more about the Principles of Green Chemistry.
refractive index
n20/D 1.437 (lit.)
bp
84-85 °C/25 mmHg (lit.)
density
0.837 g/mL at 20 °C
0.83 g/mL at 25 °C (lit.)
application(s)
flavors and fragrances
Documentation
see Safety & Documentation for available documents
food allergen
no known allergens
fragrance allergen
no known allergens
greener alternative category
Organoleptic
mushroom; musty; earthy
SMILES string
CCCCCC(O)C=C
InChI
1S/C8H16O/c1-3-5-6-7-8(9)4-2/h4,8-9H,2-3,5-7H2,1H3
InChI key
VSMOENVRRABVKN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Application
- Fluctuation of flavor quality in roasted duck: The consequences of raw duck preform′s repetitive freeze-thawing.: This study explores how repetitive freeze-thaw cycles of raw duck preforms influence the flavor quality of roasted duck, examining specific volatile compounds including 1-Octen-3-ol and its impact on sensory attributes (Gao et al., 2024).
- A new HS-SPME-GC-MS analytical method to identify and quantify compounds responsible for changes in the volatile profile in five types of meat products during aerobic storage at 4 °C.: This research introduces a novel analytical approach using HS-SPME-GC-MS to track changes in volatile profiles, including the role of 1-Octen-3-ol, across different meat products under specific storage conditions (Acquaticci et al., 2024).
- Comprehensive investigation on the dynamic changes of volatile metabolites in fresh scent green tea during processing by GC-E-Nose, GC-MS, and GC × GC-TOFMS.: This detailed study assesses how volatile metabolites like 1-Octen-3-ol evolve during the processing of fresh scent green tea, utilizing advanced gas chromatography techniques (Wang et al., 2024).
- Insights into "wheat aroma": Analysis of volatile components in wheat grains cultivated in saline-alkali soil.: This article examines the ′wheat aroma′ by analyzing volatile components, including 1-Octen-3-ol, in wheat grains grown in challenging saline-alkali soils, highlighting the adaptative traits of these crops (Sun et al., 2024).
- Demonstrating the Applicability of Proton Transfer Reaction Mass Spectrometry to Quantify Volatiles Emitted by the Mycoparasitic Fungus Trichoderma atroviride in Real Time: Monitoring of Trichoderma-Based Biopesticides.: This study demonstrates the use of proton transfer reaction mass spectrometry to quantify volatile organic compounds, including 1-Octen-3-ol, emitted in real-time by the fungus Trichoderma atroviride, used in biopesticide applications (Lochmann et al., 2024).
Other Notes
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Acute Tox. 4 Inhalation - Skin Irrit. 2 - Skin Sens. 1
Storage Class Code
6.1C - Combustible, acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 2
Flash Point(F)
154.4 °F - closed cup
Flash Point(C)
68 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service