790435
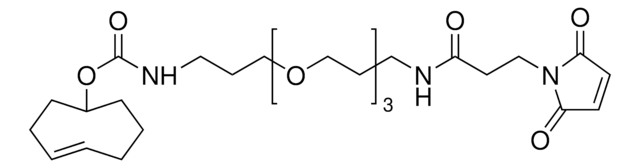
TCO PEG4 succinimidyl ester
Synonym(s):
trans-Cyclooctene-PEG4-NHS
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
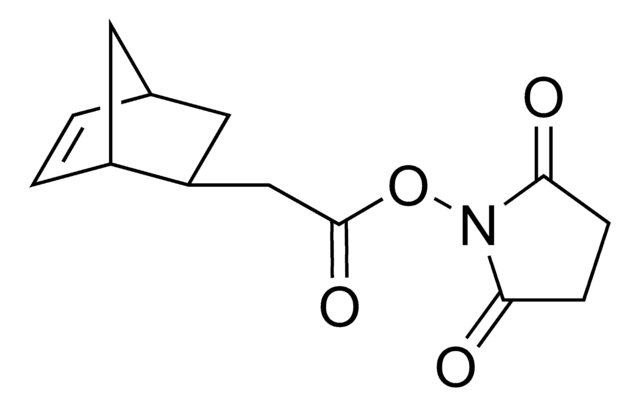
Empirical Formula (Hill Notation):
C24H38N2O10
Molecular Weight:
514.57
MDL number:
UNSPSC Code:
12352108
PubChem Substance ID:
NACRES:
NA.22
form:
liquid
Recommended Products
form
liquid
Quality Level
reaction suitability
reaction type: click chemistry
reagent type: linker
functional group
NHS ester
storage temp.
−20°C
SMILES string
O=C(NCCOCCOCCOCCOCCC(ON1C(CCC1=O)=O)=O)OC2CCC/C=C/CC2
InChI
1S/C24H38N2O10/c27-21-8-9-22(28)26(21)36-23(29)10-12-31-14-16-33-18-19-34-17-15-32-13-11-25-24(30)35-20-6-4-2-1-3-5-7-20/h1-2,20H,3-19H2,(H,25,30)/b2-1+
InChI key
ZKPMRASGLDBKPF-OWOJBTEDSA-N
Application
Succinimidyl ester/NHS (amine reactive) functionalized trans-cyclooctene derivative for incorporation of the cyclooctene moiety into amine containing compounds or biomolecules. Trans-cyclooctenes are useful in strain-promoted, copper-free, click chemistry cycloaddition reactions with 1, 2, 4, 5-tetrazines. This cyclooctene will react with tetrazine functionalized compounds or biomolecules, without the need for a catalyst, to result in a stable covalent linkage. The 4+2 inverse electron demand Diels-Alder cycloaddition between trans-cyclooctene and tetrazines is the fastest biologically compatible ligation technology reported, and has had many applications in biological labeling and imaging. The PEG spacer allows for increased water solubility, less aggregation, and an increased distance between the amine to be modified and the reactive alkene.
related product
Product No.
Description
Pricing
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Mark R Karver et al.
Bioconjugate chemistry, 22(11), 2263-2270 (2011-09-29)
1,2,4,5-Tetrazines have been established as effective dienes for inverse electron demand [4 + 2] Diels-Alder cycloaddition reactions with strained alkenes for over 50 years. Recently, this reaction pair combination has been applied to bioorthogonal labeling and cell detection applications; however
Neal K Devaraj et al.
Bioconjugate chemistry, 19(12), 2297-2299 (2008-12-05)
Bioorthogonal tetrazine cycloadditions have been applied to live cell labeling. Tetrazines react irreversibly with the strained dienophile norbornene forming dihydropyrazine products and dinitrogen. The reaction is high yielding, selective, and fast in aqueous media. Her2/neu receptors on live human breast
Melissa L Blackman et al.
Journal of the American Chemical Society, 130(41), 13518-13519 (2008-09-19)
Described is a bioorthogonal reaction that proceeds with unusually fast reaction rates without need for catalysis: the cycloaddition of s-tetrazine and trans-cyclooctene derivatives. The reactions tolerate a broad range of functionality and proceed in high yield in organic solvents, water
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service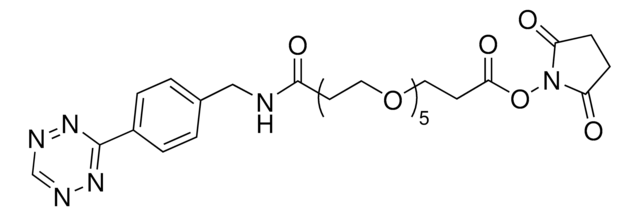




![9-Oxabicyclo[6.1.0]non-4-ene 95%](/deepweb/assets/sigmaaldrich/product/structures/328/338/c44d0a8d-81ab-4a17-81bb-aebb26a006e7/640/c44d0a8d-81ab-4a17-81bb-aebb26a006e7.png)
![(1R,8S,9s)-Bicyclo[6.1.0]non-4-yn-9-ylmethyl N-succinimidyl carbonate for Copper-free Click Chemistry](/deepweb/assets/sigmaaldrich/product/structures/969/022/d6776082-2f7a-47c7-bcd4-3830dac0fb7d/640/d6776082-2f7a-47c7-bcd4-3830dac0fb7d.png)