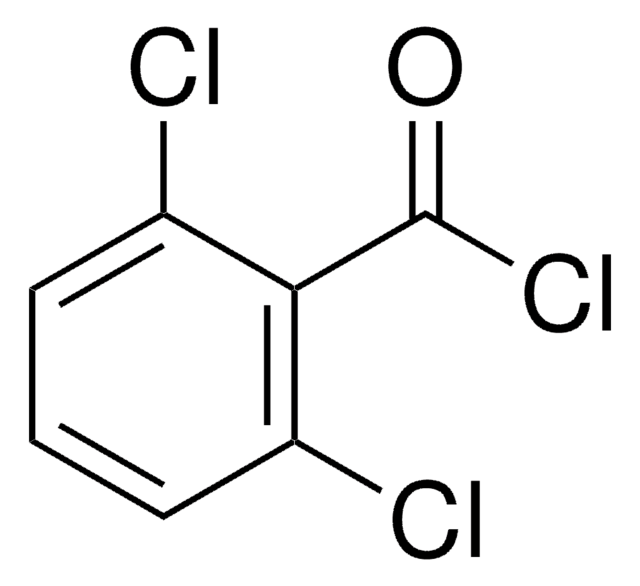
345504
2,4,6-Trichlorobenzoyl chloride
97%
Synonym(s):
2,4,6-Trichlorobenzoyl chloride
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
Cl3C6H2COCl
CAS Number:
Molecular Weight:
243.90
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.5754 (lit.)
bp
107-108 °C/6 mmHg (lit.)
density
1.561 g/mL at 25 °C (lit.)
functional group
acyl chloride
chloro
SMILES string
ClC(=O)c1c(Cl)cc(Cl)cc1Cl
InChI
1S/C7H2Cl4O/c8-3-1-4(9)6(7(11)12)5(10)2-3/h1-2H
InChI key
OZGSEIVTQLXWRO-UHFFFAOYSA-N
Related Categories
Application
2,4,6-Trichlorobenzoyl chloride may be used in the preparation of:
- γ-lactone and δ-lactone
- aliphatic aromatic anhydrides, required for the synthesis of amphiphilic hyaluronan
- mixed anhydride, required for the synthesis of angelate esters
- synthesis of both spongistatin 1 and spongistatin 2
- large-ring lactones in high yields.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Improved preparation of angelate esters.
Hartmann B, et al.
Tetrahedron Letters, 32(38), 5077-5080 (1991)
Fieser, M.
Reagents for Organic Synthesis, 16, 353-353 (1992)
Machiko Ono et al.
Chemical & pharmaceutical bulletin, 61(4), 464-470 (2013-04-03)
Alkaline hydrolysis of 4-hydroxy- or/and 5-hydroxy-(2E)-alkenoate followed by acid treatment gave the corresponding (2E)-alkenoic acids which were subjected to lactone formation reaction without further purification. The crude acids were treated with 2,4,6-trichlorobenzoyl chloride in pyridine to afford γ-lactone or δ-lactone
Michael T Crimmins et al.
Journal of the American Chemical Society, 124(20), 5661-5663 (2002-05-16)
The total synthesis of spongistatin 1 (1) and spongistatin 2 (2) has been achieved through an advanced-stage intermediate. The synthesis is highlighted by a highly convergent assembly of the four key fragments (the C1-C15 AB fragment 2, the C16-C28 CD
Gloria Huerta-Angeles et al.
Carbohydrate polymers, 111, 883-891 (2014-07-20)
The present work describes a novel and efficient method of synthesis of amphiphilic hyaluronan (HA) by esterification with alkyl fatty acids. These derivatives were synthesized under mild aqueous and well controlled conditions using mixed aliphatic aromatic anhydrides. These anhydrides characterized
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service



![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)