All Photos(1)
About This Item
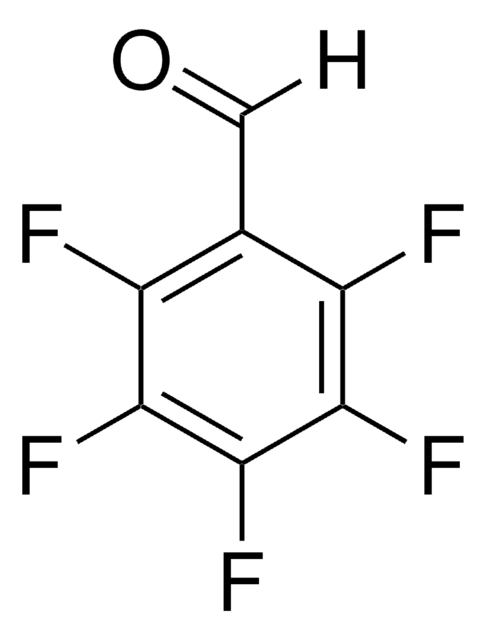
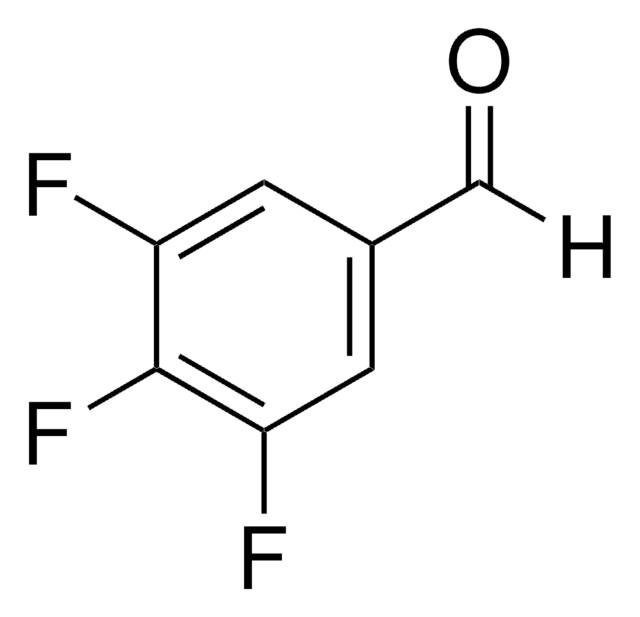
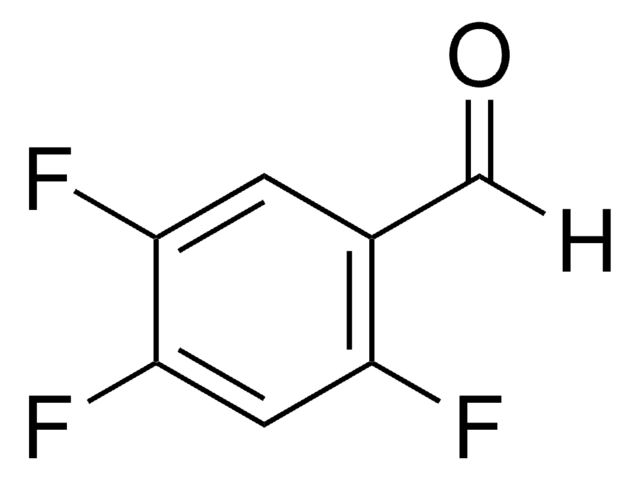
Linear Formula:
HC6F4CHO
CAS Number:
Molecular Weight:
178.08
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.469 (lit.)
bp
178 °C (lit.)
density
1.525 g/mL at 25 °C (lit.)
functional group
aldehyde
fluoro
SMILES string
Fc1cc(F)c(F)c(C=O)c1F
InChI
1S/C7H2F4O/c8-4-1-5(9)7(11)3(2-12)6(4)10/h1-2H
InChI key
YIRYOMXPMOLQSO-UHFFFAOYSA-N
General description
2,3,5,6-Tetrafluorobenzaldehyde is a polysubstituted benzaldehyde and was evaluated as a substrate of PmHNL (Prunus mume hydroxynitrile lyase). Reaction of 2,3,5,6-tetrafluorobenzaldehyde with dipyrromethane was reported.
Application
2,3,5,6-Tetrafluorobenzaldehyde was used in the preparation of 1,3-bis(2,4,6-trimethylphenyl)-2-(2,3,5,6-tetrafluorophenyl)imidazolidine and 1,3-dimethyl-2-(2,3,5,6-tetrafluorophenyl)imidazolidine.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
165.2 °F - closed cup
Flash Point(C)
74 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A new (R)-hydroxynitrile lyase from< i> Prunus mume</i>: asymmetric synthesis of cyanohydrins.
Nanda S, et al.
Tetrahedron, 61(46), 10908-10916 (2005)
Effects of aldehyde or dipyrromethane substituents on the reaction course leading to meso-substituted porphyrins.
Geier III, et al.
Tetrahedron, 60(50), 11435-11444 (2004)
Gregory W Nyce et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 10(16), 4073-4079 (2004-08-19)
The synthesis of N-heterocyclic carbene (NHC) adducts by condensation of diamines with appropriately substituted benzaldehydes is described. This simplified approach provides the NHC adduct without first having to generate the carbene followed by its protection. These adducts undergo thermal deprotection
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service