104426
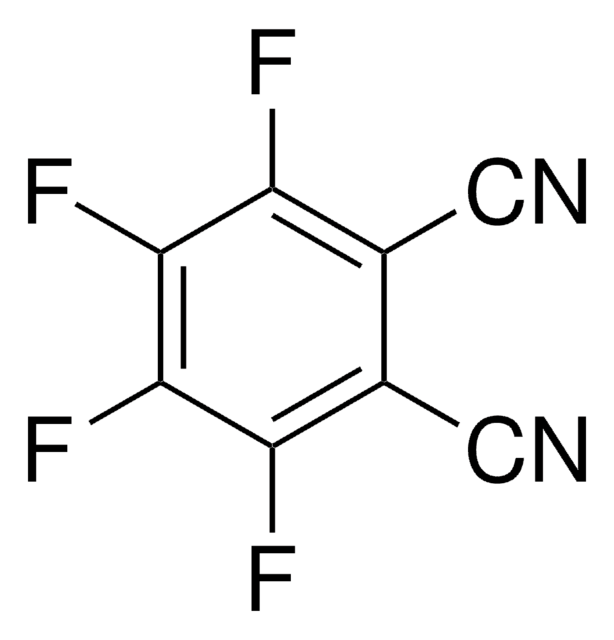
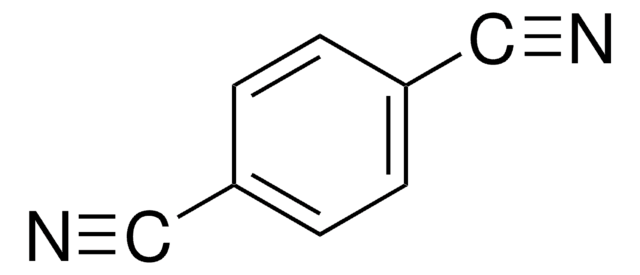
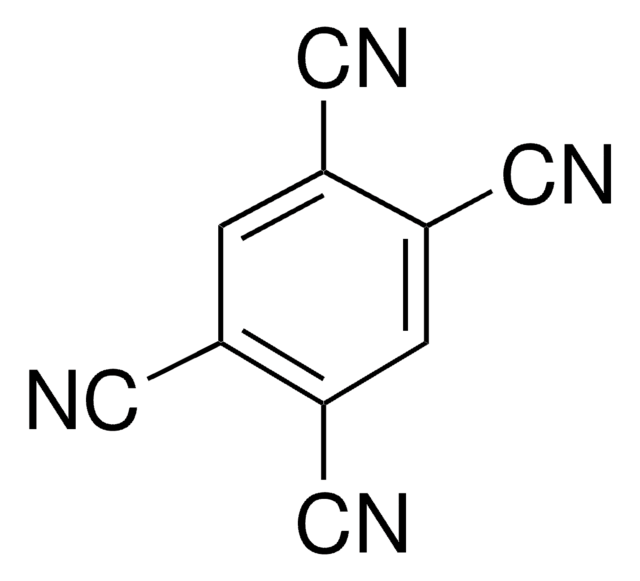
Tetrafluoroterephthalonitrile
99%
Synonym(s):
2,3,5,6-Tetrafluoro-1,4-dicyanobenzene
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C6F4-1,4-(CN)2
CAS Number:
Molecular Weight:
200.09
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
mp
197-199 °C (lit.)
solubility
acetone: soluble
SMILES string
Fc1c(F)c(C#N)c(F)c(F)c1C#N
InChI
1S/C8F4N2/c9-5-3(1-13)6(10)8(12)4(2-14)7(5)11
InChI key
PCRSJGWFEMHHEW-UHFFFAOYSA-N
General description
Tetrafluoroterephthalonitrile reacts with alkyl Grignard reagents to form corresponding 4-alkyltetrafluorobenzonitriles. Tetrafluoroterephthalonitrile acts as a four-electron donor ligand and forms tungsten(II)η2-nitrile complexes.
Application
Tetrafluoroterephthalonitrile can be used in the synthesis of Polymers of Intrinsic Microporosity (PIM). Tetrafluoroterephthalonitrile was used to study ultraviolet (UV)-rearranged polymers of PIM-1 membranes for efficient separation of H2 and CO2 .
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Rhys Short et al.
Chemical communications (Cambridge, England), 47(24), 6822-6824 (2011-05-19)
Microporous polymers derived from the 1,2- and 1,4-regioisomers of di(3',4'-dihydroxyphenyl)tetraphenylbenzene have very different properties with the former being composed predominantly of cyclic oligomers whereas the latter is of high molar mass suitable for the formation of robust solvent-cast films of
The mono-alkyldecyanation of tetrafluoroterephthalonitrile by reaction with Grignard reagents.
Milner DJ.
Journal of Organometallic Chemistry, 302(2), 147-152 (1986)
Mohammed N Alnajrani et al.
Scientific reports, 10(1), 794-794 (2020-01-23)
Traces of antibiotics within domestic and industrial effluents have toxic impact on human health as well as surrounding flora and fauna. Potential increase in antibiotic resistance of microorganisms is likely to rise due to the incomplete removal of antibiotics by
Donya Ramimoghadam et al.
Chemphyschem : a European journal of chemical physics and physical chemistry, 20(12), 1613-1623 (2019-05-09)
There has been recent interest in polymers of intrinsic microporosity (PIMs) for solid-state hydrogen-storage materials; however, the gas-sorption properties and conditions for hydrogen uptake are relatively unexplored. PIM-1 has been synthesised using the condensation reaction between 3,3,3,3-tetramethyl-1,1-spirobisindane-5,5,6,6-tetraol and 2,3,5,6-tetrafluorophthalonitrile as
Allergic contact dermatitis from tetrafluoroterephthalonitrile and TFX diamine.
A J Carmichael et al.
Contact dermatitis, 20(3), 233-234 (1989-03-01)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service