408220
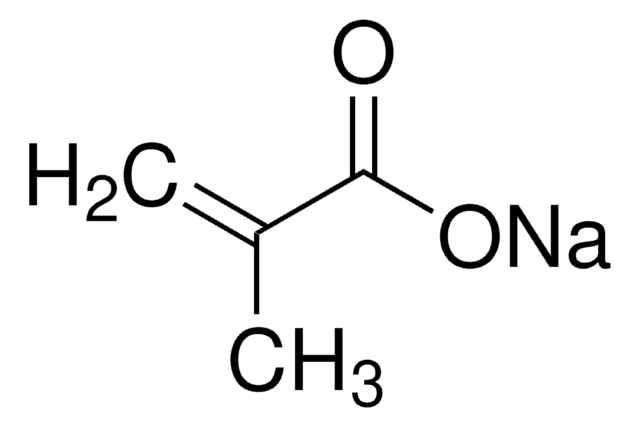
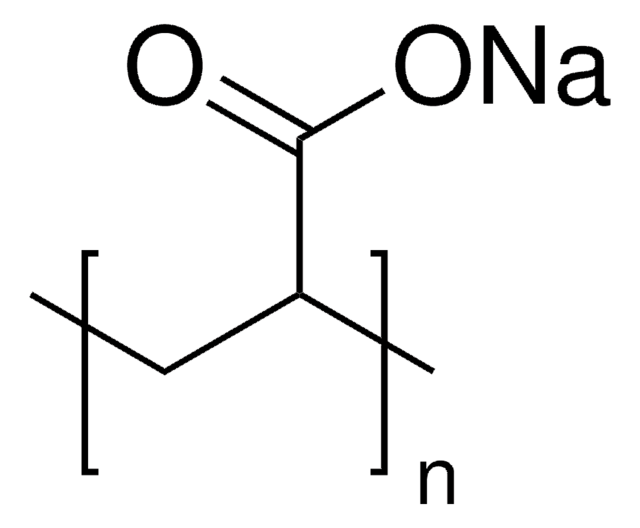
Sodium acrylate
97%
Synonym(s):
Acrylic acid sodium salt
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
H2C=CHCO2Na
CAS Number:
Molecular Weight:
94.04
EC Number:
MDL number:
UNSPSC Code:
12162002
PubChem Substance ID:
NACRES:
NA.23
Recommended Products
Quality Level
Assay
97%
form
powder
mp
>300 °C (lit.)
SMILES string
[Na+].[O-]C(=O)C=C
InChI
1S/C3H4O2.Na/c1-2-3(4)5;/h2H,1H2,(H,4,5);/q;+1/p-1
InChI key
NNMHYFLPFNGQFZ-UHFFFAOYSA-M
Looking for similar products? Visit Product Comparison Guide
General description
Sodium acrylate (SA) is a metal salt that can be prepared by an acid-base reaction between sodium hydroxide and acrylic acid. It can be used in the preparation of poly(sodium acrylate) using bulk, solution, emulsion, and suspension polymerization techniques. It results in the formation of a water soluble polymer that can be used in a variety of industries and personal care applications.
Application
SA can be used in tuning the grain size of iron oxide microparticles for potential applications in biological assaying and in chemical sensors. It can be used as a reducing and capping agent on gold nanoparticles which can be used in the biological application.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Aquatic Acute 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Polymerization of sodium acrylate in inverse-suspension stabilized by sorbitan fatty esters
Omidian H, et al.
European Polymer Journal, 39(5), 1013-1018 (2003)
Tuning the grain size and particle size of superparamagnetic Fe3O4 microparticles
Xuan S, et al.
Chemistry of Materials, 21(21), 5079-5087 (2009)
Preparation of reactive mineral powders used for poly (sodium acrylate) composite superabsorbents
Lee W and Chen Y
Journal of Applied Polymer Science, 97(3), 855-861 (2005)
Aggregative growth in the size-controlled growth of monodispersed gold nanoparticles
Njoki PN, et al.
Langmuir, 26(16), 13622-13629 (2010)
Ka I Lee et al.
International journal of biological macromolecules, 95, 826-832 (2016-11-29)
Silk was modified via in situ polymerization of two monomers acrylamide and sodium acrylate by swelling in an effective LiBr dissolution system. Swelling of natural silks in LiBr solutions of low concentration was clearly observed under optical microscope, and their
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service