426016
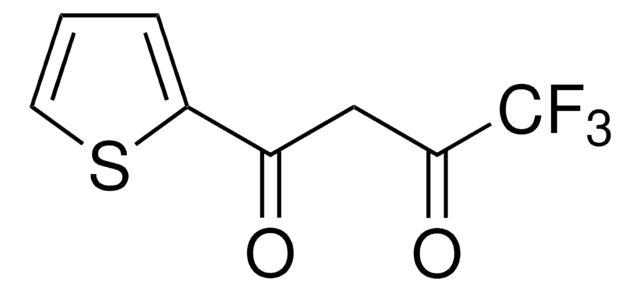
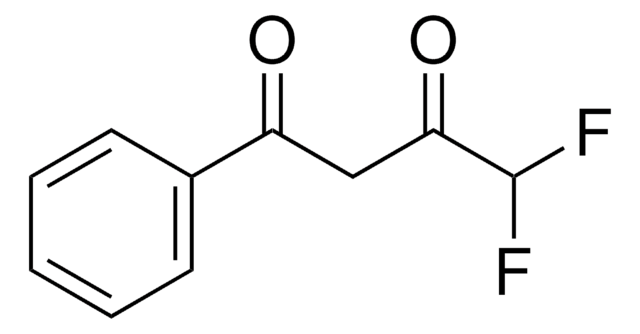
4,4,4-Trifluoro-1-(2-furyl)-1,3-butanedione
99%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C8H5F3O3
CAS Number:
Molecular Weight:
206.12
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
liquid
refractive index
n20/D 1.528 (lit.)
bp
203 °C (lit.)
mp
19-21 °C (lit.)
density
1.391 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
FC(F)(F)C(=O)CC(=O)c1ccco1
InChI
1S/C8H5F3O3/c9-8(10,11)7(13)4-5(12)6-2-1-3-14-6/h1-3H,4H2
InChI key
OWLPCALGCHDBCN-UHFFFAOYSA-N
General description
4,4,4-Trifluoro-1-(2-furyl)-1,3-butanedione (furoyltrifluoroacetone, FTFA) is a β-diketone. Its cytotoxic activity against human cultured tumor and normal cells has been evaluated. Reports suggest that 4,4,4-trifluoro-1-(2-furyl)-1,3-butanedione partially inhibits the oxidation of ferrocyanide in ETP (electron transport particles) isolated from beef heart mitochondria. Its reaction with N,N,N′,N′-tetramethylalkyl diamines to form ionic adducts has been investigated. The conformational analysis of the enol and keto form of FTFA has been reported.
Application
4,4,4-Trifluoro-1-(2-furyl)-1,3-butanedione (tfa) may be used in the following studies:
- As capping ligand in the synthesis of [Eu(tfa)3]2bpm complexes (bpm=2,2′-bipyrimidine).
- As reagent in the multistep synthesis of [13CD2]benzylamine.
- As reagent in the synthesis of 3-trifluoromethyl-2-arylcarbonylquinoxaline 1,4-di-N-oxide derivatives by reacting with corresponding benzofurazan oxides.
- In the efficient syntheses of perfluoroalkyl substituted azoles.
- Synthesis of 2-arylcarbonyl-3-trifluoromethylquinoxaline 1,4-di-N-oxide derivatives.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
188.6 °F - closed cup
Flash Point(C)
87 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
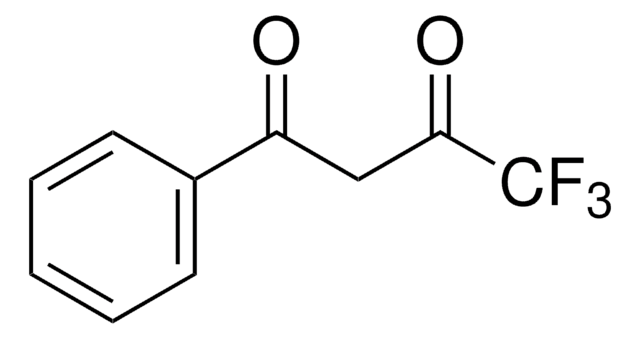
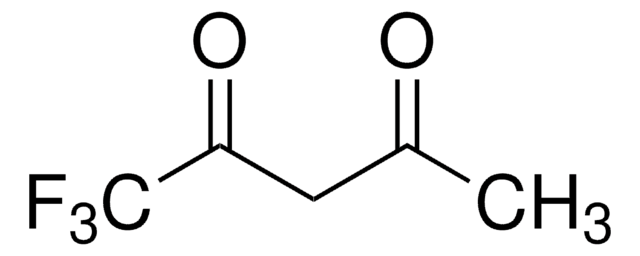
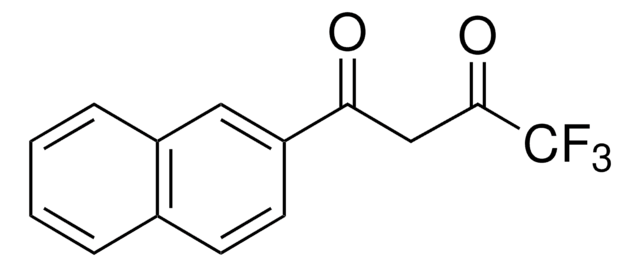
Customers Also Viewed
X-ray structure and temperature dependent luminescent properties of two bimetallic europium complexes.
Swavey S, et al.
Polyhedron, 27(3), 1061-1069 (2008)
An efficient entry to perfluoroalkyl substituted azoles starting from β-perfluoroalkyl-β-dicarbonyl compounds.
Bravo P, et al.
Tetrahedron, 50(29), 8827-8836 (1994)
Kensuke Nakano et al.
Anticancer research, 24(2B), 711-717 (2004-05-27)
A variety of beta-diketones were evaluated for their cytotoxic profiles against oral human normal and tumor cells. Among 22 compounds (BD1-22) tested, the cytotoxicity of 3-formylchromone (BD17) (CC50=7.8 microg/mL) against human oral squamous cell carcinoma (HSC-2) cells was higher than
Conformation, structure, intramolecular hydrogen bonding, and vibrational assignment of 4,4, 4-trifluoro-1-(2-furyl)-1,3-butanedione.
Tayyari SF, et al.
Journal of Molecular Structure, 8882(1), 153-167 (2008)
A Mutlib et al.
Drug metabolism and disposition: the biological fate of chemicals, 29(10), 1296-1306 (2001-09-19)
The role of gamma-glutamyltranspeptidase (GGT) in transferring glutamate from endogenous glutathione (GSH) to the benzylamine moiety of a compound, such as 1-[3-(aminomethyl)phenyl]-N-[3-fluoro-2'-(methylsulfonyl)-[1,1'-biphenyl]-4-yl]-3-(trifluoromethyl)-1H-pyrazole-5-carboxamide (DPC 423), is described. Studies were performed with structurally related analogs of DPC 423 to demonstrate that this
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service