1299200
USP
Griseofulvin Permeability Diameter
United States Pharmacopeia (USP) Reference Standard
동의어(들):
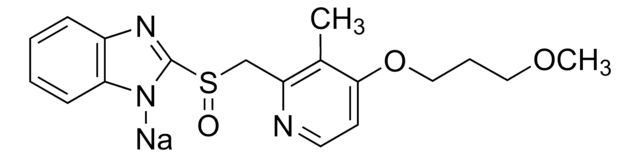
Griseofulvin, (2S)-trans-7-Chloro-2′,4,6-trimethoxy-6′-methylspiro(benzofuran-2[3H],1′-[2]cyclohexene)-3,4′-dione
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C17H17ClO6
CAS Number:
Molecular Weight:
352.77
Beilstein:
95226
MDL number:
UNSPSC 코드:
41116107
PubChem Substance ID:
NACRES:
NA.24
추천 제품
Grade
pharmaceutical primary standard
API family
griseofulvin
제조업체/상표
USP
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-8°C
SMILES string
ClC1=C(O[C@@]2(C(OC)=CC(C[C@H]2C)=O)C3=O)C3=C(OC)C=C1OC
InChI
1S/C17H17ClO6/c1-8-5-9(19)6-12(23-4)17(8)16(20)13-10(21-2)7-11(22-3)14(18)15(13)24-17/h6-8H,5H2,1-4H3/t8-,17+/m1/s1
InChI key
DDUHZTYCFQRHIY-RBHXEPJQSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Griseofulvin Permeability Diameter USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia. Also, for use with USP monograph such as Griseofulvin
분석 메모
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
기타 정보
Sales restrictions may apply.
관련 제품
제품 번호
설명
가격
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Carc. 2 - Repr. 1B - Skin Sens. 1
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
Mark Sacchetti
Journal of pharmaceutical sciences, 103(9), 2772-2783 (2013-12-12)
Pharmaceutical materials, crystalline and amorphous, sorb water from the atmosphere, which affects critical factors in the development of drugs, such as the selection of drug substance crystal form, compatibility with excipients, dosage form selection, packaging, and product shelf-life. It is
Damir Elmar Zecevic et al.
European journal of pharmaceutics and biopharmaceutics : official journal of Arbeitsgemeinschaft fur Pharmazeutische Verfahrenstechnik e.V, 87(2), 264-270 (2014-04-29)
Many upcoming drug candidates are pH-dependent poorly soluble weak bases in the pH range of the gastrointestinal tract. This often leads to a high in vivo variability and bioavailability issues. Aiming to overcome these limitations, the design of solid dispersions
Dipy M Vasa et al.
Journal of pharmaceutical sciences, 103(9), 2911-2923 (2014-05-16)
Fifteen model drugs were quenched from 3:1 (w/w) mixtures with polyethylene glycol 4000 (PEG4000). The resulting solids were characterized using powder X-ray diffraction (PXRD), analysis of pair distribution function-transformed PXRD data (where appropriate), hot-stage polarized light microscopy, and differential scanning
Kayo Yuminoki et al.
Journal of pharmaceutical sciences, 103(11), 3772-3781 (2014-09-12)
In this study, we reported the application of Povacoat®, a hydrophilic polyvinylalcohol copolymer, as a dispersion stabilizer of nanoparticles of poorly water-soluble compounds. In addition, the influence of aggregation of the nanoparticles on their solubility and oral absorption was studied.
Niraj S Trasi et al.
The journal of physical chemistry. B, 118(33), 9974-9982 (2014-07-31)
Amorphous forms of drugs are increasingly being used to deliver poorly water-soluble compounds. Therefore, understanding the magnitude and origin of differences in crystallization kinetics is highly important. The goal of this study was to better understand the factors that influence
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.