추천 제품
Grade
pharmaceutical primary standard
API family
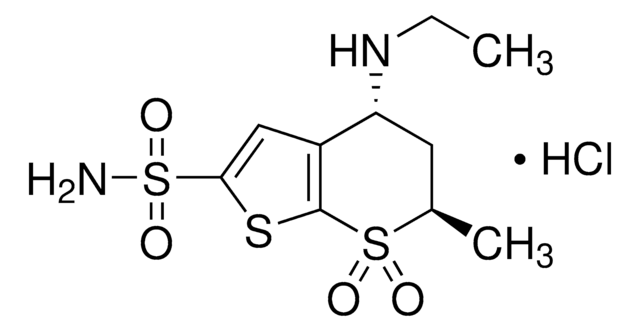
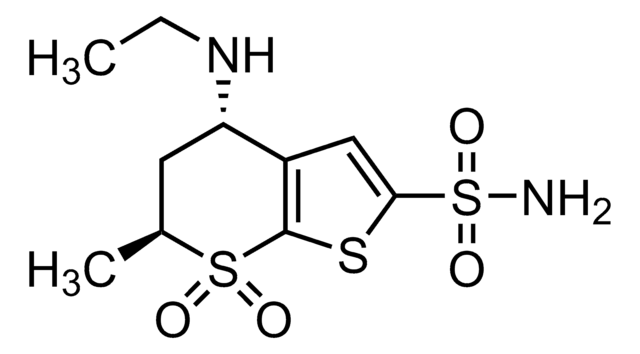
dorzolamide
제조업체/상표
USP
응용 분야
pharmaceutical (small molecule)
형식
neat
SMILES string
Cl.CCN[C@H]1C[C@H](C)S(=O)(=O)c2sc(cc12)S(N)(=O)=O
InChI
1S/C10H16N2O4S3.ClH/c1-3-12-8-4-6(2)18(13,14)10-7(8)5-9(17-10)19(11,15)16;/h5-6,8,12H,3-4H2,1-2H3,(H2,11,15,16);1H/t6-,8-;/m0./s1
InChI key
OSRUSFPMRGDLAG-QMGYSKNISA-N
유전자 정보
human ... CA2(760)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Dorzolamide hydrochloride USP reference standard, intended for use in specified quality tests and assays as specified in the USP compendia. Also, for use with USP monographs such as:
- Dorzolamide Hydrochloride and Timolol Maleate Ophthalmic Solution
- Dorzolamide Hydrochloride Ophthalmic Solution
분석 메모
These products are for test and assay use only. They are not meant for administration to humans or animals and cannot be used to diagnose, treat, or cure diseases of any kind.
기타 정보
Sales restrictions may apply.
관련 제품
제품 번호
설명
가격
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - STOT RE 2
표적 기관
Central nervous system,Gastrointestinal tract,Bone,Blood,Bladder
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
M F Sugrue
Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics, 12(3), 363-376 (1996-01-01)
Dorzolamide hydrochloride (S,S-5,6-dihydro-4H-4-ethylamino-6-methylthieno [2,3-b]thiopyran-2-sulfonamide-7,7-dioxide HCl; MK-507; L-671,152) is a water-soluble, potent inhibitor of human carbonic anhydrase isoenzymes II and IV in vitro, the respective IC50 values being 0.18 nM and 6.9 nM. In contrast, it was found to be a
Göktuğ Seymenoğlu et al.
Journal of glaucoma, 24(2), 111-116 (2013-06-29)
To compare the efficacy of fixed combinations of dorzolamide-timolol (FCDT) and brimonidine-timolol (FCBT) in patients with intraocular pressure (IOP) elevations after intravitreal triamcinolone acetonide (IVTA) injections. This was a prospective, randomized, open-label study. Patients who received IVTA injections due to
Masamichi Fukuda et al.
Journal of ocular pharmacology and therapeutics : the official journal of the Association for Ocular Pharmacology and Therapeutics, 31(1), 57-60 (2014-10-11)
We investigated the transcorneal penetration of commercial ophthalmic formulations containing timolol maleate in rabbit eyes. One drop (30 μL) of each ophthalmic solution (Xalacom(®), DuoTrav(®), Cosopt(®), and Timoptol(®)) was administered to the conjunctival sac of the rabbits' eyes and the
Azza A Hasan
Pharmaceutical development and technology, 19(6), 748-754 (2013-08-24)
The objective of this work was to formulate and characterize non-ionic surfactant vesicles (niosomes) as an ocular carrier of dorzolamide hydrochloride (Dorzo); one of the antiglaucoma drugs. Niosomes were prepared of Cholesterol (Chol) with sorbitan monoesters (Span 20, 40, 60)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.