추천 제품
애플리케이션
For trypsin digestion of peptides, use a ratio of about 1:100 to 1:20 for trypsin:peptide. The typical use for this product is in removing adherent cells from a culture surface. The concentration of trypsin necessary to dislodge cells from their substrate is dependent primarily on the cell type and the age of the culture. Trypsins have also been used for the re-suspension of cells during cell culture, in proteomics research for digestion of proteins and in various in-gel digestions. Additional applications include assessing crystallization by membrane-based techniques and in a study to determine that protein folding rates and yields can be limited by the presence of kinetic traps.
생화학적/생리학적 작용
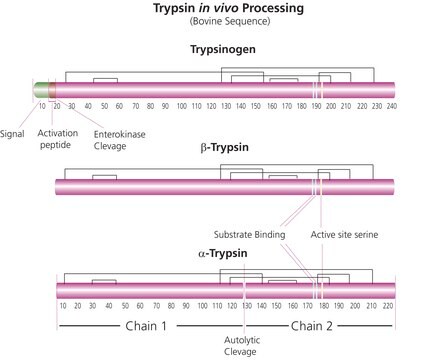
Trypsin cleaves peptides on the C-terminal side of lysine and arginine residues. The rate of hydrolysis of this reaction is slowed if an acidic residue is on either side of the cleavage site and hydrolysis is stopped if a proline residue is on the carboxyl side of the cleavage site. The optimal pH for trypsin activity is 7-9. Trypsin can also act to cleave ester and amide linkages of synthetic derivatives of amino acids. EDTA is added to trypsin solutions as a chelating agent that neutralizes calcium and magnesium ions that obscure the peptide bonds on which trypsin acts. Removing these ions increases the enzymatic activity.
Serine protease inhibitors, including DFP, TLCK, APMSF, AEBSEF, and aprotinin, amongst others, will inhibit Trypsin.
Serine protease inhibitors, including DFP, TLCK, APMSF, AEBSEF, and aprotinin, amongst others, will inhibit Trypsin.
주의사항
This product should be stored frozen at -20°C.
단위 정의
One BAEE unit will produce a A253 of 0.001 per minute at pH 7.6 at 25°C using BAEE as a substrate.
제조 메모
This is 25 g/L porcine trypsin solution in 0.9% sodium chloride.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Resp. Sens. 1 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
Guoqian He et al.
Apoptosis : an international journal on programmed cell death, 21(4), 390-403 (2016-02-18)
Autophagic (type II) cell death has been suggested to play pathogenetic roles in cerebral ischemia. Growth arrest and DNA damage response 45b (Gadd45b) has been shown to protect against rat brain ischemia injury through inhibiting apoptosis. However, the relationship between
Sei-Jung Lee et al.
Food and chemical toxicology : an international journal published for the British Industrial Biological Research Association, 87, 12-22 (2015-12-04)
This study was carried out to investigate the anti-inflammatory potentials of a 38 kDa glycoprotein isolated from Styrax japonica Siebold et al Zuccarini (SJSZ glycoprotein). We found that SJSZ glycoprotein has concentration-dependent scavenging activity against DPPH and hydroxyl radicals in the cell-free
Volker Kroehne et al.
Frontiers in cellular neuroscience, 11, 284-284 (2017-09-30)
Endogenous oligodendrocyte progenitor cells (OPCs) are a promising target to improve functional recovery after spinal cord injury (SCI) by remyelinating denuded, and therefore vulnerable, axons. Demyelination is the result of a primary insult and secondary injury, leading to conduction blocks
Yochai Wolf et al.
Cell, 179(1), 219-235 (2019-09-17)
Although clonal neo-antigen burden is associated with improved response to immune therapy, the functional basis for this remains unclear. Here we study this question in a novel controlled mouse melanoma model that enables us to explore the effects of intra-tumor
Guo-Qian He et al.
Molecular medicine reports, 22(6), 5083-5094 (2020-11-12)
Autophagy and the ubiquitin proteasome system (UPS) are two major protein degradation pathways involved in brain ischemia. Autophagy can compensate for UPS impairment‑induced cellular dysfunction. HECT, UBA and WWE domain containing E3 ubiquitin protein ligase 1 (Huwe1), an E3 ubiquitin ligase, serves critical roles
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.