T0261
Thiamphenicol
동의어(들):
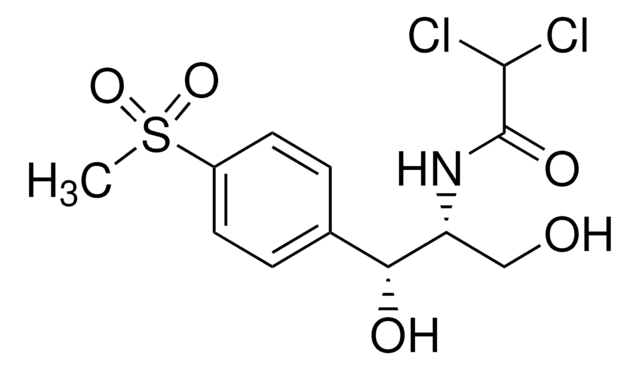
Raceophenidol, Thiophenicol, D-threo-2,2-Dichloro-N-(β-hydroxy-α-[hydroxymethyl]-4-[methylsulfonyl]phenethyl)acetamide
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C12H15Cl2NO5S
CAS Number:
Molecular Weight:
356.22
Beilstein:
2819542
EC Number:
MDL number:
UNSPSC 코드:
51284303
PubChem Substance ID:
NACRES:
NA.85
추천 제품
양식
powder
Quality Level
색상
white to off-white
solubility
ethanol: soluble 50 mg/mL
항생제 활성 스펙트럼
Gram-negative bacteria
Gram-positive bacteria
동작 모드
protein synthesis | interferes
SMILES string
CS(=O)(=O)c1ccc(cc1)[C@@H](O)[C@@H](CO)NC(=O)C(Cl)Cl
InChI
1S/C12H15Cl2NO5S/c1-21(19,20)8-4-2-7(3-5-8)10(17)9(6-16)15-12(18)11(13)14/h2-5,9-11,16-17H,6H2,1H3,(H,15,18)/t9-,10-/m1/s1
InChI key
OTVAEFIXJLOWRX-NXEZZACHSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Chemical structure: phenicole
애플리케이션
Thiamphenicol is an antibiotic that has been used to treat chancroid in men and uncomplicated gonorrhea. It is used in studies of bacterial protein synthesis at the level of peptidyl transferase activity associated with the 23S rRNA of the 50S ribosomal subunit. It is used to study chloraniphenicol-thiamphenicol-resistance and the use of fluorinated analogs when resistance is encountered.
생화학적/생리학적 작용
Thiamphenicol inhibits mitochondrial protein synthesis of proteins such as cytochrome c oxidase.
포장
1G,5G,25G
기타 정보
Keep container tightly closed in a dry and well-ventilated place.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
D R Rezende et al.
Food additives & contaminants. Part A, Chemistry, analysis, control, exposure & risk assessment, 29(4), 559-570 (2012-01-14)
A validated method based on European and Brazilian legislation is reported. It is applicable to the simultaneous determination of chloramphenicol (CAP) and florfenicol (FF) by LC-MS/MS in liquid milk, milk powder and bovine muscle. The chromatographic analysis is completed in
Weixin Tao et al.
Applied and environmental microbiology, 78(17), 6295-6301 (2012-07-04)
Chloramphenicol and florfenicol are broad-spectrum antibiotics. Although the bacterial resistance mechanisms to these antibiotics have been well documented, hydrolysis of these antibiotics has not been reported in detail. This study reports the hydrolysis of these two antibiotics by a specific
Aptasensing of chloramphenicol in the presence of its analogues: reaching the maximum residue limit.
Sanaz Pilehvar et al.
Analytical chemistry, 84(15), 6753-6758 (2012-06-26)
A novel, label-free folding induced aptamer-based electrochemical biosensor for the detection of chloramphenicol (CAP) in the presence of its analogues has been developed. CAP is a broad-spectrum antibiotic that has lost its favor due to its serious adverse toxic effects
V P Syriopoulou et al.
Antimicrobial agents and chemotherapy, 19(2), 294-297 (1981-02-01)
We evaluated the in vitro antimicrobial activity of Sch 24893, Sch 25298, and Sch 25393, three novel analogs of chloramphenicol and thiamphenicol. All of the analogs had minimal inhibitory concentrations of less than or equal to 10 micrograms/ml for 18
T E Tupasi et al.
The British journal of venereal diseases, 59(3), 172-175 (1983-06-01)
The use of cefuroxime and thiamphenicol in uncomplicated gonococcal infection was studied in 562 women confined to a clinic to preclude reinfection before cultural confirmation of cure. Cefuroxime was as effective as spectinomycin in the treatment of infections due to
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.