SML2483
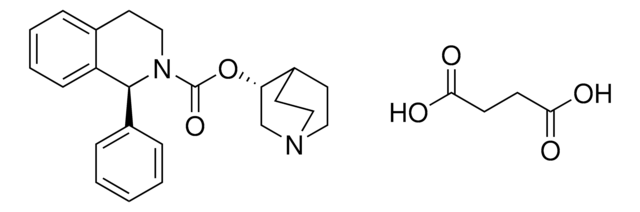
Fesoterodine fumarate
≥93% (HPLC)
동의어(들):
2-Methylpropanoic acid-2-[(1R)-3-[bis(1-methylethyl)amino]-1-phenylpropyl]-4-(hydroxymethyl)phenyl ester, fumarate salt
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C26H37NO3 · C4H4O4
CAS Number:
Molecular Weight:
527.65
MDL number:
UNSPSC 코드:
12352200
NACRES:
NA.77
추천 제품
분석
≥93% (HPLC)
양식
powder
색상
white to beige
solubility
H2O: 2 mg/mL, clear
저장 온도
2-8°C
SMILES string
O=C(C(C)C)OC1=C([C@@H](C2=CC=CC=C2)CCN(C(C)C)C(C)C)C=C(CO)C=C1.OC(/C=C/C(O)=O)=O
InChI
1S/C26H37NO3.C4H4O4/c1-18(2)26(29)30-25-13-12-21(17-28)16-24(25)23(22-10-8-7-9-11-22)14-15-27(19(3)4)20(5)6;5-3(6)1-2-4(7)8/h7-13,16,18-20,23,28H,14-15,17H2,1-6H3;1-2H,(H,5,6)(H,7,8)/b;2-1+/t23-;/m1./s1
InChI key
MWHXMIASLKXGBU-RNCYCKTQSA-N
생화학적/생리학적 작용
Fesoterodine fumarate is a prodrug of 5-hydroxymethyl tolterodine (5-HMT), which a muscarinic M2 and M3-receptor antagonist with antispasmodic activity, and is also the active metabolite of tolterodine. Fesoterodine fumarate is used clinically for the treatment of overactive bladder (OAB).
Muscarinic M2 and M3-receptor antagonist; Anti-spasmodic
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Irrit. 2 - Repr. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
Xavier Biardeau et al.
PloS one, 12(1), e0169694-e0169694 (2017-01-07)
In spinal cord injury, onset of detrusor overactivity (DO) is detrimental for quality of life (incontinence) and renal risk. Prevention has only been achieved with complex sophisticated electrical neuromodulation techniques. To assess the efficacy of early fesoterodine fumarate (FF) administration
Shizuo Yamada et al.
Pharmacology & therapeutics, 189, 130-148 (2018-05-02)
Antimuscarinic agents are now widely used as the pharmacological therapy for overactive bladder (OAB) because neuronal (parasympathetic nerve) and non-neuronal acetylcholine play a significant role for the bladder function. In this review, we will highlight basic and clinical aspects of
Xavier Gamé et al.
European journal of pharmacology, 833, 155-157 (2018-05-29)
Fesoterodine (as one of three drugs: dutasteride, finasteride and fesoterodine) was classified B (beneficial) by LUTS-FORTA 2014, indicating that it is a medicinal product with proven or obvious efficacy in the elderly, with limited side effects and/or safety concerns. A
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.