SML1415
VE-821
≥98% (HPLC)
동의어(들):
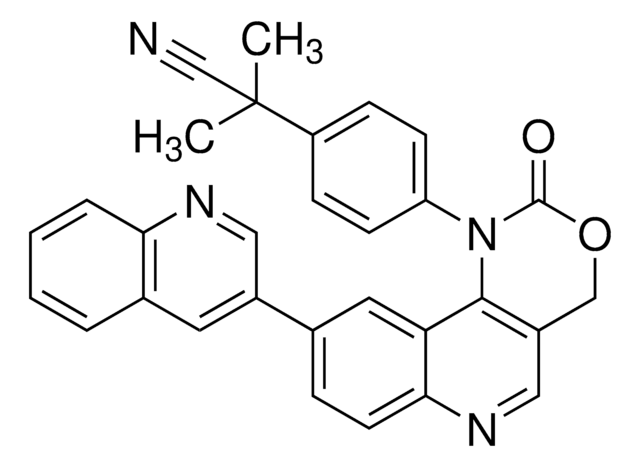
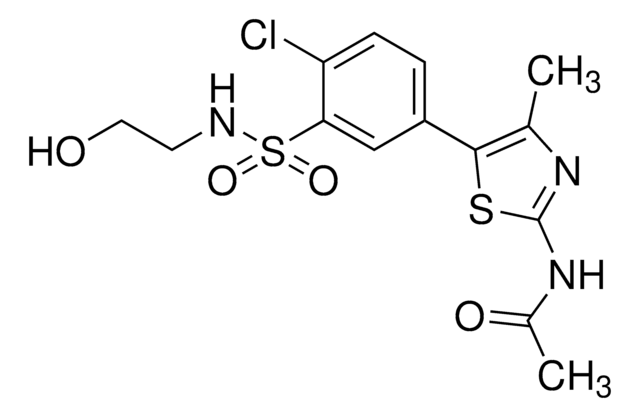
3-Amino-6-(4-(methylsulfonyl)phenyl)-N-phenylpyrazine-2-carboxamide, 3-Amino-6-[4-(methylsulfonyl)phenyl]-N-phenyl-2-pyrazinecarboxamide, VE821
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C18H16N4O3S
CAS Number:
Molecular Weight:
368.41
MDL number:
UNSPSC 코드:
12352200
PubChem Substance ID:
NACRES:
NA.77
추천 제품
Quality Level
분석
≥98% (HPLC)
양식
powder
색상
white to beige
solubility
DMSO: ≥10 mg/mL, clear
저장 온도
−20°C
SMILES string
CS(C(C=C1)=CC=C1C2=NC(C(NC3=CC=CC=C3)=O)=C(N)N=C2)(=O)=O
InChI
1S/C18H16N4O3S/c1-26(24,25)14-9-7-12(8-10-14)15-11-20-17(19)16(22-15)18(23)21-13-5-3-2-4-6-13/h2-11H,1H3,(H2,19,20)(H,21,23)
InChI key
DUIHHZKTCSNTGM-UHFFFAOYSA-N
애플리케이션
VE-821 has been used as an inhibitor of ATM- and Rad3-related (ATR) protein in human cancer cells.
생화학적/생리학적 작용
VE-821 is a ATP-competitive inhibitor of ATR.
VE-821 is a potent ATP-competitive inhibitor of the DNA damage response (DDR) kinase Ataxia telangiectasia-mutated (ATM) and ATM- and Rad3-related (ATR) with a Ki of 13 nM. VE-821 has minimal cross-reactivity against the related PIKKs ATM, DNA-dependent protein kinase (DNA-PK), mTOR and PI3-kinase-γ (Ki of 16 μM, 2.2 μM, >1 μM and 3.9 μM, respectively) and against a large panel of unrelated protein kinases. VE-821 used alone caused death in a large fraction of cancer cell populations and also showed strong synergy with genotoxic agents. VE-821 increased sensitivity of cells to radiation and also sensitized cancer cells to a variety of chemotherapeutic agents.
기타 정보
VE-821 has been expertly reviewed and recommended by the Chemical Probes Portal. For more information, please visit the VE-821 probe summary on the Chemical Probes Portal website.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
Anastazja Poczta et al.
Scientific reports, 9(1), 14135-14135 (2019-10-03)
The present study investigated the effect of cladribine (CLA) and six of its derivatives containing a formamidine group at position 6 (CLA-FDM, CLA-FPAZ, CLA-FPIR, CLA-FPIP, CLA-FHEX, and CLA-FMOR) on acute promyelocytic, lymphoblastic, and acute monocytic leukemia cells. The role of
Human cancer cells utilize mitotic DNA synthesis to resist replication stress at telomeres regardless of their telomere maintenance mechanism
Ozgun Ozer, et al.
Oncotarget null
Tingting Li et al.
Aging, 12(14), 14791-14807 (2020-07-21)
Protection of telomere 1 (POT1), the telomeric single-stranded DNA (ssDNA)-binding protein in the shelterin complex, has been implicated in the DNA damage response, tumorigenesis and aging. Telomere dysfunction induced by telomere deprotection could accelerate cellular senescence in primary human cells.
Aaron Mendez-Bermudez et al.
Molecular cell, 70(3), 449-461 (2018-05-05)
Hard-to-replicate regions of chromosomes (e.g., pericentromeres, centromeres, and telomeres) impede replication fork progression, eventually leading, in the event of replication stress, to chromosome fragility, aging, and cancer. Our knowledge of the mechanisms controlling the stability of these regions is essentially
Camelia Mocanu et al.
Cell reports, 39(3), 110701-110701 (2022-04-21)
Mitotic DNA synthesis (MiDAS) has been proposed to restart DNA synthesis during mitosis because of replication fork stalling in late interphase caused by mild replication stress (RS). Contrary to this proposal, we find that cells exposed to mild RS in
Global Trade Item Number
| SKU | GTIN |
|---|---|
| SML1415-25MG | 4061832451831 |
| SML1415-5MG | 4061832451848 |
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.