추천 제품
Quality Level
분석
≥97% (HPLC)
양식
powder
색상
white to beige
solubility
DMSO: 10 mg/mL, clear
저장 온도
−20°C
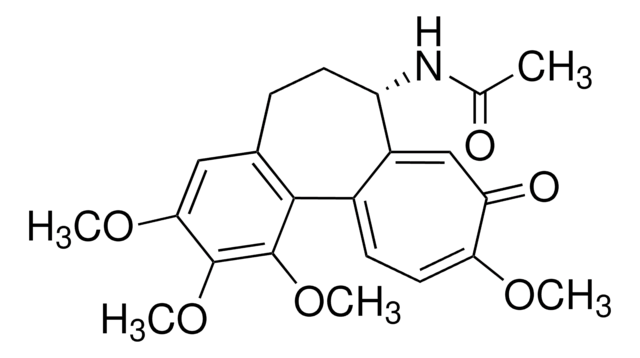
SMILES string
S(C)c1[c](cc2c(cc1)c3c(cc(c(c3OC)OC)OC)CC[C@@H]2NC(=O)C)=O
InChI
1S/C22H25NO5S/c1-12(24)23-16-8-6-13-10-18(26-2)21(27-3)22(28-4)20(13)14-7-9-19(29-5)17(25)11-15(14)16/h7,9-11,16H,6,8H2,1-5H3,(H,23,24)/t16-/m0/s1
InChI key
CMEGANPVAXDBPL-INIZCTEOSA-N
생화학적/생리학적 작용
Thiocolchicine is an antimitotic alkaloid and apoptosis inducer that inhibits tubulin polymerization and microtubule assembly.
Thiocolchicine is an antimitotic alkaloid and apoptosis inducer.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 1 Inhalation - Acute Tox. 2 Oral - Eye Dam. 1 - Muta. 1B
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
Bruno Danieli et al.
The Journal of organic chemistry, 71(7), 2848-2853 (2006-03-25)
A dynamic combinatorial library of thiocolchicine-podophyllotoxin derivatives based on the disulfide bond exchange reaction is described. The influence of a biological target on the composition of the reaction mixture has been demonstrated. Use of high-resolution ESI mass spectrometry to evaluate
R De Vincenzo et al.
Oncology research, 11(3), 145-152 (1999-10-20)
Three new 7-0-substituted deacetamidothiocolchicine derivatives have been evaluated for their antitumor activity against various human tumor cell lines, some of which express the multidrug resistance (MDR) phenotype, for their impact on the cell cycle and their binding to tubulin. Colchicine
L Sun et al.
Journal of medicinal chemistry, 36(10), 1474-1479 (1993-05-14)
Three series of novel thiocolchicine analogs, N-acyl-, N-aroyl-, and N-(substituted benzyl)-deacetylthiocolchicinoids, have been synthesized and evaluated for their cytotoxicity against various tumor cell lines, especially solid tumor cell lines, and for their inhibitory effects on tubulin polymerization in vitro. Most
R Brecht et al.
Bioorganic & medicinal chemistry, 8(3), 557-562 (2000-03-25)
Several B-ring variations of O-methyl androbiphenyline (8), newly accessible from (-)-(M,7S)-colchicine via photooxygenation and subsequent endoperoxide-transformation, were synthesized and evaluated for their inhibitory effects on tubulin assembly in vitro. The amino-allocolchicinoid (9), a key compound in this study, was transformed
Recent progress in structure-activity relationship studies on the anticancer drug colchicine and its analogues.
Xian-dao Pan et al.
Yao xue xue bao = Acta pharmaceutica Sinica, 37(10), 821-827 (2003-02-06)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.