추천 제품
분석
≥97% (HPLC)
형태
powder
색상
white to off-white
solubility
DMSO: >10 mg/mL
H2O: insoluble
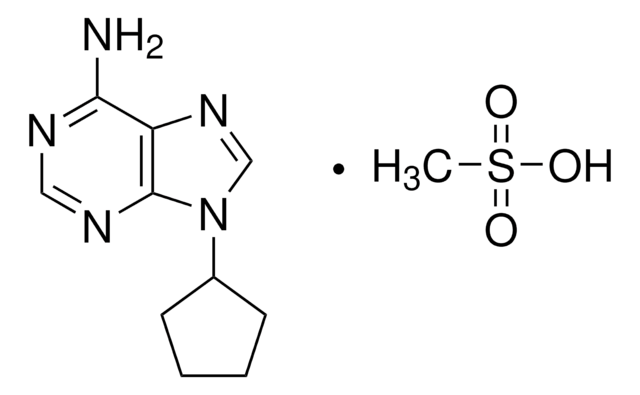
SMILES string
Nc1ncnc2n(cnc12)C3CCCO3
InChI
1S/C9H11N5O/c10-8-7-9(12-4-11-8)14(5-13-7)6-2-1-3-15-6/h4-6H,1-3H2,(H2,10,11,12)
InChI key
UKHMZCMKHPHFOT-UHFFFAOYSA-N
유전자 정보
human ... ADCY1(107) , ADCY2(108) , ADCY3(109) , ADCY4(196883) , ADCY5(111) , ADCY6(112) , ADCY7(113) , ADCY8(114) , ADCY9(115)
rat ... Adora1(29290) , Adora2a(25369)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
생화학적/생리학적 작용
특징 및 장점
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
관련 콘텐츠
Cyclic nucleotides, including cyclic AMP (cAMP), cyclic GMP (cGMP) and cyclic ADP-ribose, have been extensively studied as second messengers of intracellular events initiated by activation of GPCRs. cAMP modifies cell function in all eukaryotic cells, principally through the activation of cAMP-dependent protein kinase (PKA), but also through cAMP-gated ion channels and guanine nucleotide exchange factors directly activated by cAMP.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.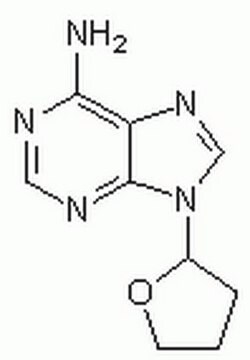



![1H-[1,2,4]Oxadiazolo[4,3-a]quinoxalin-1-one powder](/deepweb/assets/sigmaaldrich/product/structures/764/715/605dc5a5-0864-471b-a71e-bd2aa6553c1d/640/605dc5a5-0864-471b-a71e-bd2aa6553c1d.png)