추천 제품
Quality Level
분석
≥98% (HPLC)
양식
solid
광학 활성
[α]/D -8 to -13°, c = 1 in chloroform-d
색상
white
solubility
DMSO: >5 mg/mL
H2O: insoluble
저장 온도
−20°C
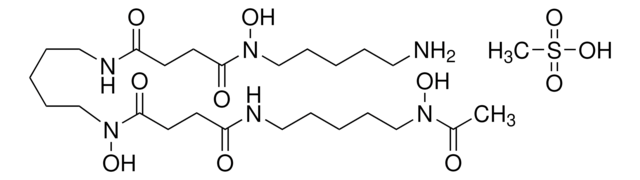
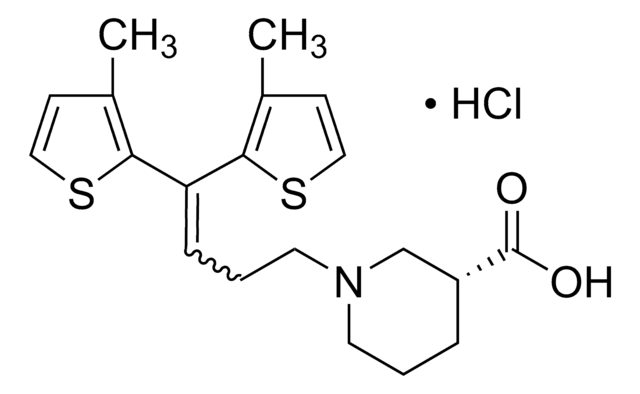
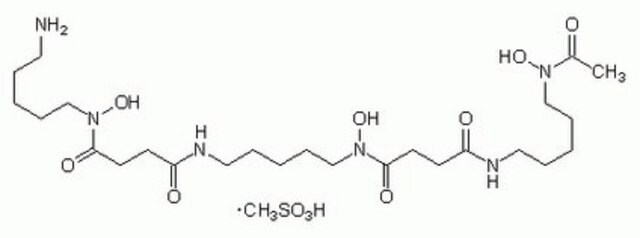
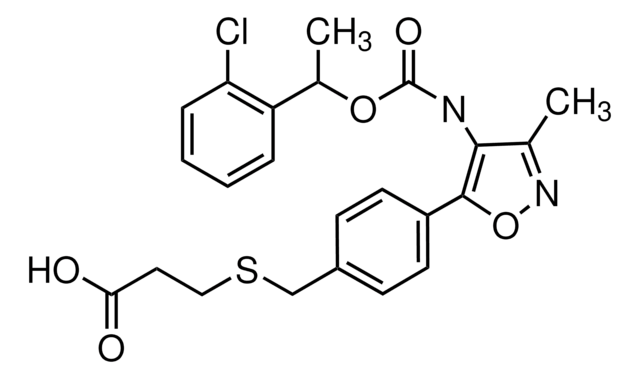
SMILES string
COc1ccc(cc1)C(OCCN2CCC[C@@H](C2)C(O)=O)(c3ccc(OC)cc3)c4ccc(OC)cc4
InChI
1S/C30H35NO6/c1-34-26-12-6-23(7-13-26)30(24-8-14-27(35-2)15-9-24,25-10-16-28(36-3)17-11-25)37-20-19-31-18-4-5-22(21-31)29(32)33/h6-17,22H,4-5,18-21H2,1-3H3,(H,32,33)/t22-/m0/s1
InChI key
VDLDUZLDZBVOAS-QFIPXVFZSA-N
생화학적/생리학적 작용
GABA transport inhibitor, showing selectivity for GAT-3 and GAT-2.
특징 및 장점
This compound is a featured product for Neuroscience research. Click here to discover more featured Neuroscience products. Learn more about bioactive small molecules for other areas of research at sigma.com/discover-bsm.
This compound is featured on the GABA Transporters page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
N O Dalby
Neuropharmacology, 39(12), 2399-2407 (2000-09-07)
The present study examines the effect of tiagabine (a selective inhibitor of GABA transporter 1, GAT-1), SNAP-5114 (a semi-selective inhibitor of rat GAT-3/mouse GAT4) and NNC 05-2045 (a non-selective GABA uptake inhibitor) in modulating GABA levels in the hippocampus and
Maria Ek Lie et al.
Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism, 38(1), 166-173 (2017-11-18)
Brain ischemia triggers excitotoxicity and cell death, yet no neuroprotective drugs have made it to the clinic. While enhancing GABAergic signaling to counterbalance excitotoxicity has shown promise in animal models, clinical studies have failed. Blockade of GABA transporters (GATs) offers
Jonas M Fuks et al.
PLoS pathogens, 8(12), e1003051-e1003051 (2012-12-14)
During acute infection in human and animal hosts, the obligate intracellular protozoan Toxoplasma gondii infects a variety of cell types, including leukocytes. Poised to respond to invading pathogens, dendritic cells (DC) may also be exploited by T. gondii for spread
L A Borden
Neurochemistry international, 29(4), 335-356 (1996-10-01)
gamma-Aminobutyric acid (GABA) is the major inhibitory neurotransmitter in the mammalian brain. GABA is cleared from the synaptic cleft by specific, high-affinity, sodium- and chloride-dependent transporters, which are thought to be located on presynaptic terminals and surrounding glial cells. While
Daniel J McGrail et al.
Cancer cell, 37(3), 371-386 (2020-02-29)
Deficient DNA mismatch repair (dMMR) induces a hypermutator phenotype that can lead to tumorigenesis; however, the functional impact of the high mutation burden resulting from this phenotype remains poorly explored. Here, we demonstrate that dMMR-induced destabilizing mutations lead to proteome
관련 콘텐츠
DISCOVER Bioactive Small Molecules for Neuroscience
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.