추천 제품
종 반응성
human
포장
kit of 96 wells (12 strips x 8 wells)
기술
ELISA: suitable
입력
sample type plasma
sample type cell culture supernatant(s)
sample type serum
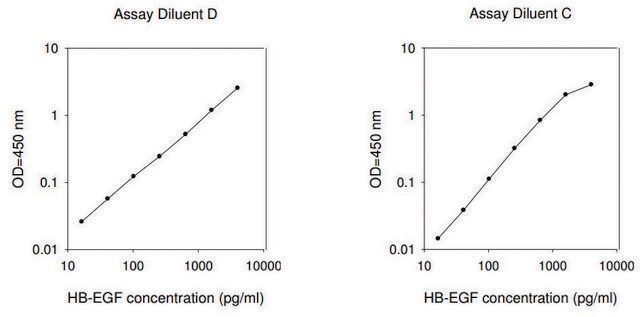
assay range
inter-assay cv: <12%
intra-assay cv: <10%
sensitivity: 2 pg/mL
standard curve range: 12.29-3000 pg/mL
검출 방법
colorimetric
배송 상태
wet ice
저장 온도
−20°C
유전자 정보
human ... HAVCR1(26762)
일반 설명
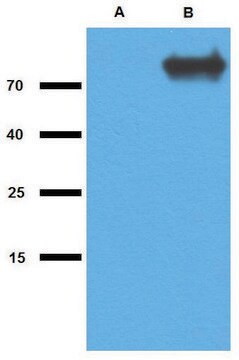
The antibody pair provided in this kit recognizes Human Hepatitis A Virus Cellular Receptor 1.
애플리케이션
For research use only. Not for use in diagnostic procedures.
Please refer to the attached General ELISA KIT Procedure (sandwich, competitive & Indirect ELISA)
Please refer to the attached General ELISA KIT Procedure (sandwich, competitive & Indirect ELISA)
기타 정보
A sample Certificate of Analysis is available for this product.
Please type the word sample in the text box provided for lot number.
Please type the word sample in the text box provided for lot number.
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Met. Corr. 1
Storage Class Code
8A - Combustible, corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
Kevin J Downes et al.
The Journal of antimicrobial chemotherapy, 72(1), 254-260 (2016-09-03)
Tobramycin is frequently used for treatment of bronchopneumonia in patients with cystic fibrosis (CF). Variability in tobramycin clearance (CL) is high in this population with few reliable approaches to guide dosing. We sought to evaluate the pharmacokinetics of once-daily intravenous
Suzana Bojic et al.
Disease markers, 2018, 5064684-5064684 (2018-06-05)
The role of matrix metalloproteinase-9 (MMP-9) and tissue inhibitor of matrix metalloproteinase-1 (TIMP-1) in sepsis after major abdominal surgery and sepsis-associated organ dysfunction is unexplored. Fifty-three patients with sepsis after major abdominal surgery were compared to 50 operated and 50
Julia Neuhaus et al.
Kidney & blood pressure research, 43(5), 1563-1572 (2018-10-23)
The addition of immunosuppression to supportive care reduces proteinuria in a subset of patients with IgA nephropathy (IgAN) but is associated with an increased rate of adverse events. The present work investigates whether urinary biomarkers are able to identify subjects
Jian-Jhong Wang et al.
Critical care (London, England), 22(1), 108-108 (2018-04-28)
Acute kidney injury (AKI) after cardiovascular surgery is a serious complication. Little is known about the ability of novel biomarkers in combination with clinical risk scores for prediction of advanced AKI. In this prospectively conducted multicenter study, urine samples were
Maciej T Wybraniec et al.
Journal of interventional cardiology, 30(5), 465-472 (2017-07-08)
The study was designed to evaluate the applicability of combined assessment of urinary biomarkers and intra-renal Doppler flow indices for the prediction of contrast-induced acute kidney injury (CI-AKI) after coronary angiography/percutaneous coronary interventions (CA/PCI). This prospective observational study covered 95
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.