추천 제품
분석
≥98%
양식
powder
mp
183-184 °C (lit.)
저장 온도
2-8°C
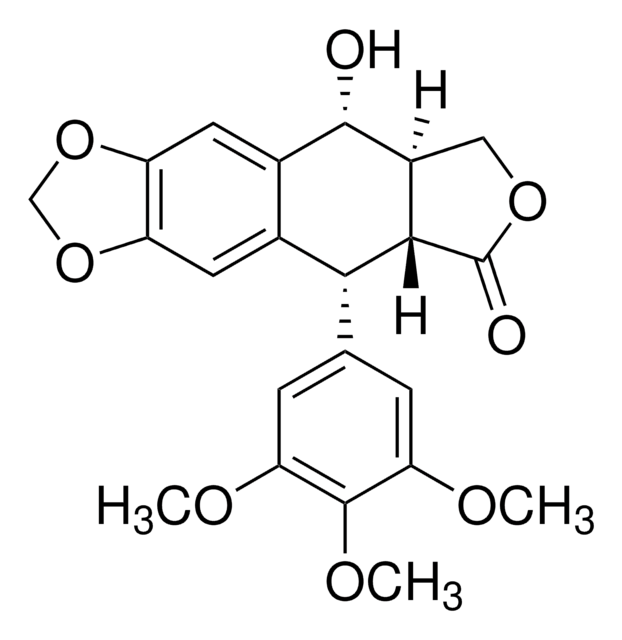
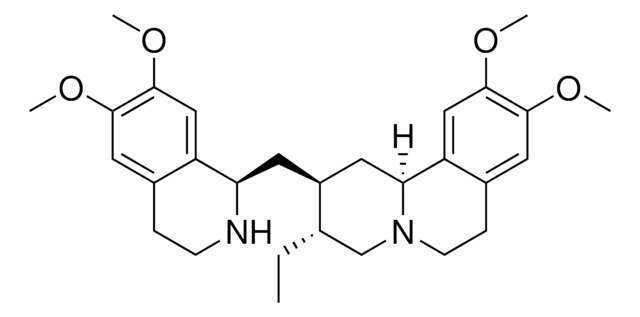
SMILES string
COc1cc(cc(OC)c1OC)[C@H]2C3C(COC3=O)[C@@H](O)c4cc5OCOc5cc24
InChI
1S/C22H22O8/c1-25-16-4-10(5-17(26-2)21(16)27-3)18-11-6-14-15(30-9-29-14)7-12(11)20(23)13-8-28-22(24)19(13)18/h4-7,13,18-20,23H,8-9H2,1-3H3/t13-,18+,19-,20-/m0/s1
InChI key
YJGVMLPVUAXIQN-XVVDYKMHSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Podophyllotoxin has been used as a reference compound for evaluating the cytotoxicity in VERO cells. It has also been used as a reference standard in high-performance liquid chromatography (HPLC).
생화학적/생리학적 작용
Inhibits microtubule assembly; antineoplastic.
Podophyllotoxin is a naturally occurring lignan obtained from resin podophyllin present in the genus Podophyllum. It exhibits antiviral and antimitotic properties. Podophyllotoxin may be used to treat anogenital warts in children and dermatological conditions caused by psoriasis vulgaris. It may also be used as a therapeutic agent to treat genital tumors, Wilms tumors, lung cancer, and lymphomas.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Inhalation - Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P2 (EN 143) respirator cartridges
이미 열람한 고객
Y Damayanthi et al.
Current medicinal chemistry, 5(3), 205-252 (1998-07-01)
Podophyllotoxin is a natural product isolated from Podophyllum peltatum and Podophyllum emodi and has long been known to possess medicinal properties. Etoposide (VP-16), a podophyllotoxin derivative, is currently in clinical use in the treatment of many cancers, particularly small cell
Diterpenoids as potential anti-malarial compounds from Andrographis paniculata
Dwivedi M, et al.
Beni-Suef University Journal of Basic and Applied Sciences, 10(1), 1-16 (2021)
Svetlana M Bakunova et al.
Journal of medicinal chemistry, 50(23), 5807-5823 (2007-10-24)
Forty three cationic bisbenzofurans were synthesized either by interaction of o-hydroxyaldehydes with alpha-halogenated ketones followed by intramolecular ring closure or by a copper- or palladium-mediated heteroannulation of substituted o-iodophenols with terminal acetylenes. In vitro antiprotozoal activities of compounds 1-43 against
Jean Fotie et al.
Journal of medicinal chemistry, 53(3), 966-982 (2010-01-06)
The current chemotherapy for second stage human African trypanosomiasis is unsatisfactory. A synthetic optimization study based on the lead antitrypanosomal compound 1,2-dihydro-2,2,4-trimethylquinolin-6-yl 3,5-dimethoxybenzoate (TDR20364, 1a) was undertaken in an attempt to discover new trypanocides with potent in vivo activity. While
Patrick Hochegger et al.
Molecules (Basel, Switzerland), 26(18) (2021-09-29)
A new series of compounds was prepared from 6-methoxyquinolin-8-amine or its N-(2-aminoethyl) analogue via Ugi-azide reaction. Their linkers between the quinoline and the tert-butyltetrazole moieties differ in chain length, basicity and substitution. Compounds were tested for their antiplasmodial activity against
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.