추천 제품
일반 설명
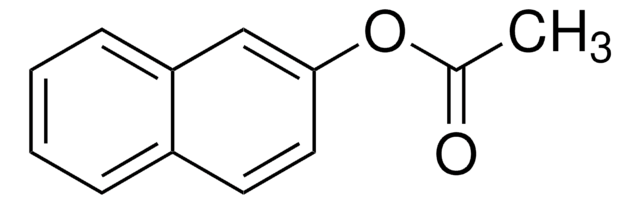
1-Naphthyl acetate is a 1-napthyl ester. Mechanism of photo-Fries rearrangement of 1-naphthyl acetate has been studied using steady state photolysis and laser flash photolysis. Kinetics of photo-Fries rearrangement of 1-napthyl acetate has been studied using steady-state, time-resolved chemically induced dynamic nuclear polarization (CIDNP) and flash photolysis methods.
애플리케이션
1-Naphthyl acetate may be used in a rapid staining method for identification of macrophages; counts by this method were confirmed by the more complex morphological criteria, by phagocytosis, and by the presence of Fc receptors. It may be employed as substrate to investigate the distribution of non-specific carboxylic esterases (EC 3.1.1) in the digestive tract of perch, Perm fliiviatilis L.
신호어
Danger
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Eye Dam. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
235.4 °F
Flash Point (°C)
113 °C
Laser flash photolysis and CIDNP studies of 1-naphthyl acetate photo-Fries rearrangement.
Gritsan NP, et al.
The Journal of Physical Chemistry, 100(11), 4448-4458 (1996)
Non-specific carboxylic esterase activity in the digestive tract of the perch, Perca fluviatilis L.
Hirji KN and Courtney WAM.
Journal of Fish Biology, 22(1), 1-7 (1983)
E F Barth et al.
Brazilian journal of biology = Revista brasleira de biologia, 78(4), 601-608 (2018-01-11)
This work describes the preliminary evaluation of cytotoxic, antimicrobial, molluscicidal, antioxidant and anticholinesterase activities from leaf (LECF) and stem bark alcoholic extracts (BECF) of the species Croton floribundus Spreng. (Euphorbiaceae), popularly known as capixingui or tapixingui. BECF presented significant toxicity
Magnetic isotope and external magnetic field effects upon the photo-Fries rearrangement of 1-naphthyl acetate.
Nakagaki R, et al.
The Journal of Physical Chemistry, 89(15), 3222-3226 (1985)
Zhuo Ma et al.
PloS one, 12(12), e0189343-e0189343 (2017-12-12)
Imidacloprid is a neonicotinoid insecticide that is effective against house fly, Musca domestica L., which is a major pest with the ability to develop resistance to insecticides. In the present study, we investigated the inheritance mode, the cross-resistance pattern and
문서
The Fries rearrangement reaction is an organic name reaction which involves the conversion of phenolic esters into hydroxyaryl ketones on heating in the presence of a catalyst. Suitable catalysts for this reaction are Brønsted or Lewis acids such as HF, AlCl3, BF3, TiCl4, or SnCl4. The Fries rearrangement reaction is an ortho, para-selective reaction, and is used in the preparation of acyl phenols. This organic reaction has been named after German chemist Karl Theophil Fries.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.