생물학적 소스
synthetic (organic)
무균
non-sterile
분석
≥98%
형태
powder
기술
inhibition assay: suitable
solubility
ethanol: 50 mg/mL, clear, greenish-yellow
주관자
Danco Laboratories
배송 상태
ambient
저장 온도
2-8°C
SMILES string
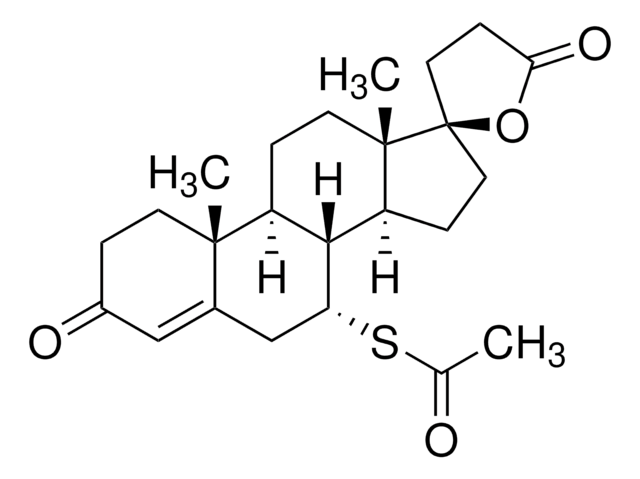
[H][C@@]12CCC3=CC(=O)CCC3=C1[C@H](C[C@@]4(C)[C@@]2([H])CC[C@@]4(O)C#CC)c5ccc(cc5)N(C)C
InChI
1S/C29H35NO2/c1-5-15-29(32)16-14-26-24-12-8-20-17-22(31)11-13-23(20)27(24)25(18-28(26,29)2)19-6-9-21(10-7-19)30(3)4/h6-7,9-10,17,24-26,32H,8,11-14,16,18H2,1-4H3/t24-,25+,26-,28-,29-/m0/s1
InChI key
VKHAHZOOUSRJNA-GCNJZUOMSA-N
유전자 정보
human ... AR(367) , ESR1(2099) , ESR2(2100) , HTR1A(3350) , HTR1B(3351) , HTR1D(3352) , HTR1E(3354) , HTR1F(3355) , HTR2A(3356) , HTR2B(3357) , HTR2C(3358) , HTR3A(3359) , HTR3B(9177) , HTR3C(170572) , HTR3D(200909) , HTR3E(285242) , HTR4(3360) , HTR5A(3361) , HTR5B(645694) , HTR6(3362) , HTR7(3363) , NR3C1(2908) , NR3C2(4306) , PGR(5241)
mouse ... Nr3c1(14815)
rat ... Nr3c1(24413) , Tat(24813)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
애플리케이션
- to induce damage against the proliferative and secretory phase of endometrial stromal cells
- to establish a preterm birth (PTB) mice model in order to study the difference in cervical ripening mechanism between term and PTBs
- to activate geneswitch gal4 in flies
- to study the effects of sex steroids on prostaglandin secretion
생화학적/생리학적 작용
특징 및 장점
신호어
Danger
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Repr. 1B
Storage Class Code
6.1C - Combustible, acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type P3 (EN 143) respirator cartridges
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.