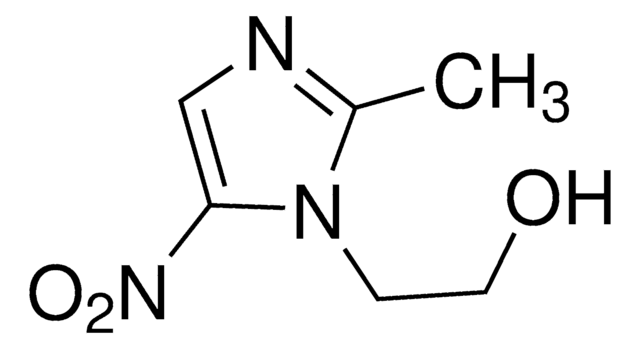
M3512
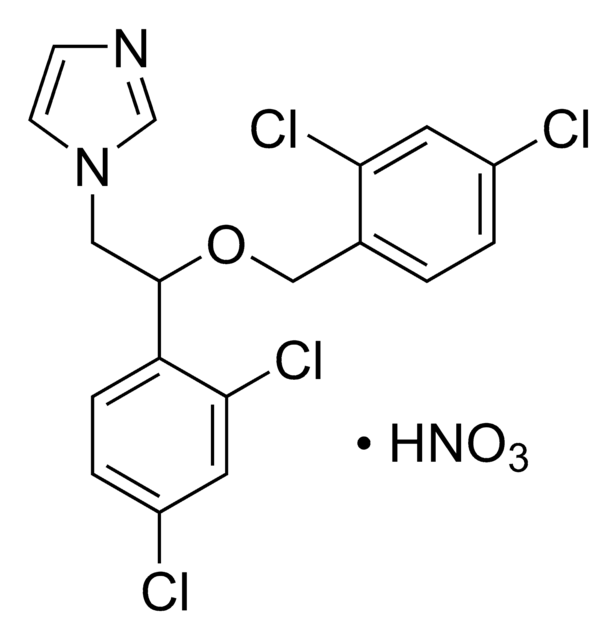
(±)-Miconazole nitrate salt
imidazole antibiotic
동의어(들):
1-(2,4-Dichloro-β-[(2,4-dichlorobenzyl)oxy]phenethyl)imidazole
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C18H14Cl4N2O · HNO3
CAS Number:
Molecular Weight:
479.14
EC Number:
MDL number:
UNSPSC 코드:
51102829
eCl@ss:
39161001
PubChem Substance ID:
NACRES:
NA.85
추천 제품
Quality Level
광학 활성
[α]/D ±0.10° (Specific Rotation (BP))
색상
white to off-white
항생제 활성 스펙트럼
fungi
mycobacteria
동작 모드
enzyme | inhibits
SMILES string
ClC1=CC(Cl)=CC=C1C(OCC2=CC=C(Cl)C=C2Cl)CN3C=CN=C3.[O-][N+](O)=O
InChI
1S/C18H14Cl4N2O.HNO3/c19-13-2-1-12(16(21)7-13)10-25-18(9-24-6-5-23-11-24)15-4-3-14(20)8-17(15)22;2-1(3)4/h1-8,11,18H,9-10H2;(H,2,3,4)
InChI key
MCCACAIVAXEFAL-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Chemical structure: imidazole
애플리케이션
Miconazole is an imidazole antifungal agent that is used topically and by intravenous infusion. It is used to inhibit cytochrome P450 and to study automated luminescence-based cytochrome P450 profiling.
생화학적/생리학적 작용
Miconazole interacts with 14-α demethylase, a cytochrome P-450 enzyme necessary for ergosterol biosynthesis. The inhibition of ergosterol results in increased cellular permeability causing leakage of cellular contents. Miconazole may also inhibit endogenous respiration, interact with phospholipids in the cell membrane, inhibit the transformation of yeasts to mycelial forms, inhibit purine uptake, and interfere with triglyceride and phospholipid biosynthesis.
제조 메모
Sparingly soluble in methanol, slightly soluble in ethanol. Very slightly soluble in water.
애플리케이션
제품 번호
설명
가격
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Skin Sens. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
Francis X Cunningham et al.
Eukaryotic cell, 6(3), 533-545 (2006-11-07)
Cyanidioschyzon merolae is considered to be one of the most primitive of eukaryotic photosynthetic organisms. To obtain insights into the origin and evolution of the pathway of carotenoid biosynthesis in eukaryotic plants, the carotenoid content of C. merolae was ascertained
Automated Luminescence-Based Cytochrome P450 Profiling Using a Simple, Elegant Robotic Platform
Brad Larson, Peter Banks, et al.
Journal of the Association for Laboratory Automation, 16, 47-55 (2011)
Michele Tonelli et al.
Bioorganic & medicinal chemistry, 16(18), 8447-8465 (2008-09-02)
Eighty-five arylazoenamines, characterized by different types of aryl and basic moieties, have been synthesized and evaluated in cell-based assays for cytotoxicity and antiviral activity against a panel of ten RNA and DNA viruses. The most commonly affected viruses were, in
Janardhanan Saravanan et al.
European journal of medicinal chemistry, 45(9), 4365-4369 (2010-06-29)
A series of 3-substituted amino-4,5-tetramethylene thieno[2,3-d] [1,2,3]-triazine-4(3H)-ones have been synthesized and characterized by UV,IR, 1H NMR, elemental and mass spectral analysis. The title compounds were evaluated for their antimicrobial activity by agar diffusion method against four bacteria and three fungi
V M Litvinov et al.
Molecular pharmaceutics, 9(10), 2924-2932 (2012-08-22)
The use of hot-melt extrusion for preparing homogeneous API-excipient mixtures is studied for miconazole-PEG-g-PVA [poly(ethylene glycol)-poly(vinyl alcohol) graft copolymer] solid dispersions with a 5 cm(3) table-top, twin-screw corotating microcompounder (DSM Xplore). Phase behavior of PEG-g-PVA, miscibility of miconazole in PEG-g-PVA
Chromatograms
application for HPLCapplication for HPLCapplication for HPLC자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.