추천 제품
생물학적 소스
synthetic (organic)
분석
≥95%
형태
powder or flakes
solid
solubility
DMSO: 4 mg/mL
저장 온도
−20°C
SMILES string
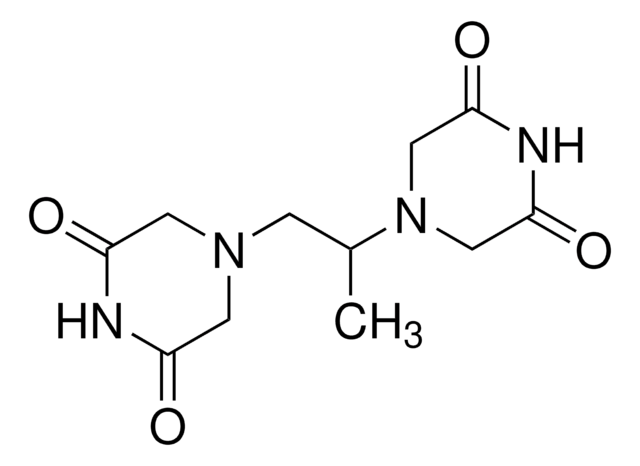
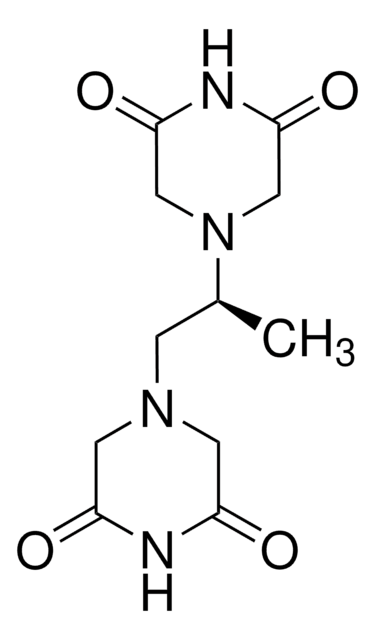
C[C@@H]([C@@H](C)N1CC(=O)NC(=O)C1)N2CC(=O)NC(=O)C2
InChI
1S/C12H18N4O4/c1-7(15-3-9(17)13-10(18)4-15)8(2)16-5-11(19)14-12(20)6-16/h7-8H,3-6H2,1-2H3,(H,13,17,18)(H,14,19,20)/t7-,8+
InChI key
OBYGAPWKTPDTAS-OCAPTIKFSA-N
일반 설명
ICRF-193 is a bisdiopiperazine derivative. It inhibits topoisomerase II by forming a non-cleavable complex.
애플리케이션
ICRF-193 has been used as a topoisomerase II (TOP2) inhibitor to treat mouse oocytes to investigate the role of TOP2 in meiosis.
생화학적/생리학적 작용
ICRF-193 helps in the enhancement of cell cycle without chromosome segregation. It is considered as an important drug for chemo-differentiation therapy against acute promyelocytic leukemia (APL). ICRF-193 serves as an inducer of differentiation between anticancer drugs.
ICRF-193 induces a G2 checkpoint that is associated with an ATR-dependent inhibition of polo-like kinase 1 (plk1) activity and a decrease in cyclin B1 phosphorylation. Induces apoptosis in several cell lines including K562 and Molt-4 cells., ICRF-193 is a topoisomerase II inhibitor, more potent against topoisomerase II-β than topoisomerase II-α, and may in addition cause DNA strand breaks.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Oral - Skin Sens. 1
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Faceshields, Gloves
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
ICRF-193, a catalytic inhibitor of DNA topoisomerase II, delays the cell cycle progression from metaphase, but not from anaphase to the G1 phase in mammalian cells
Iwai M, et al.
Febs Letters, 406(3), 267-270 (1997)
N Hajji et al.
Mutation research, 530(1-2), 35-46 (2003-10-18)
The bis-dioxopiperazine ICRF-193 has long time been considered as a pure topoisomerase II catalytic inhibitor able to exert its inhibitory effect on the enzyme without stabilization of the so-called cleavable complex formed by DNA covalently bound to topoisomerase II. In
The catalytic DNA topoisomerase II inhibitor ICRF-193 and all-trans retinoic acid cooperatively induce granulocytic differentiation of acute promyelocytic leukemia cells: candidate drugs for chemo-differentiation therapy against acute promyelocytic leukemia
Niitsu,
Experimental Hematology, 30(11), 1273-1282 (2002)
K C Huang et al.
The Journal of biological chemistry, 276(48), 44488-44494 (2001-09-29)
Antineoplastic bis(dioxopiperazine)s, such as meso-2,3-bis(2,6-dioxopiperazin-4-yl)butane (ICRF-193), are widely believed to be only catalytic inhibitors of topoisomerase II. However, topoisomerase inhibitors have little or no antineoplastic activity unless they are topoisomerase poisons, a special subclass of topoisomerase-targeting drugs that stabilize topoisomerase-DNA
Sílvia Dyson et al.
The EMBO journal, 40(1), e105393-e105393 (2020-11-07)
The juxtaposition of intracellular DNA segments, together with the DNA-passage activity of topoisomerase II, leads to the formation of DNA knots and interlinks, which jeopardize chromatin structure and gene expression. Recent studies in budding yeast have shown that some mechanism
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.