H0377
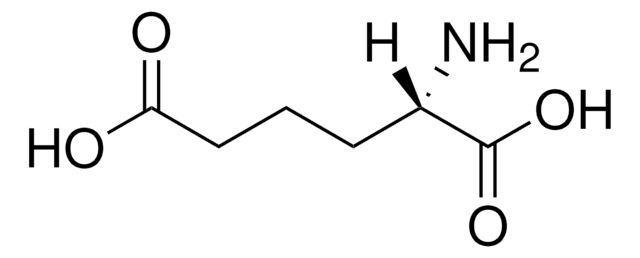
DL-5-Hydroxylysine hydrochloride
≥98% (TLC)
동의어(들):
2,6-Diamino-5-hydroxycaproic acid hydrochloride, 2,6-Diamino-5-hydroxyhexanoic acid hydrochloride
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
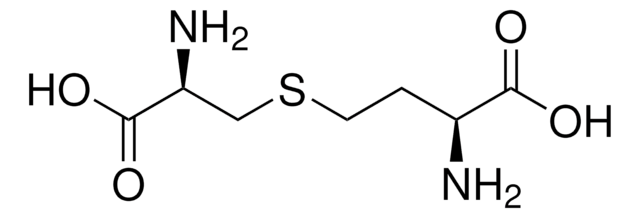
Linear Formula:
NH2CH2CH(OH)CH2CH2CH(NH2)COOH · HCl
CAS Number:
Molecular Weight:
198.65
Beilstein:
3914368
EC Number:
MDL number:
UNSPSC 코드:
12352209
PubChem Substance ID:
NACRES:
NA.26
추천 제품
제품명
DL-5-Hydroxylysine hydrochloride,
분석
≥98% (TLC)
Quality Level
양식
powder
색상
white
mp
225 °C (dec.) (lit.)
응용 분야
detection
peptide synthesis
SMILES string
Cl.NCC(O)CCC(N)C(O)=O
InChI
1S/C6H14N2O3.ClH/c7-3-4(9)1-2-5(8)6(10)11;/h4-5,9H,1-3,7-8H2,(H,10,11);1H
InChI key
MJXVOTKVFFAZQJ-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
- Characterization of acetyl-CoA: L-lysine N6-acetyltransferase, which catalyses the first step of carbon catabolism from lysine in Saccharomyces cerevisiae.: This research investigates the enzyme acetyl-CoA: L-lysine N6-acetyltransferase, which initiates the catabolism of lysine in Saccharomyces cerevisiae. Utilizing DL-5-Hydroxylysine hydrochloride, the study provides insights into the metabolic pathways and regulatory mechanisms of lysine degradation, contributing to the broader understanding of amino acid metabolism in yeast (Bode et al., 1993).
생화학적/생리학적 작용
DL-5-Hydroxylysine is a racemic mixture of D- and L- enantiomers of 5-hydroxylysine which may be used as potential target markers for radical-induced protein oxidation.
기타 정보
Mixed DL and DL-allo
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
R Bode et al.
Archives of microbiology, 160(5), 397-400 (1993-01-01)
The carbon catabolism of L-lysine starts in Saccharomyces cerevisiae with acetylation by an acetyl-CoA:L-lysine N6-acetyltransferase. The enzyme is strongly induced in cells grown on L-lysine as sole carbon source and has been purified about 530-fold. Its activity was specific for
B Morin et al.
Chemical research in toxicology, 11(11), 1265-1273 (1998-11-17)
gamma-Irradiation of several amino acids (Val, Leu, Ile, Lys, Pro, and Glu) in the presence of O2 generates hydroperoxides. We have previously isolated and characterized valine and leucine hydroperoxides, and hydroxides, and have detected these products in both isolated systems
M Droux et al.
Archives of biochemistry and biophysics, 316(1), 585-595 (1995-01-10)
Cystathionine beta-lyase, the second enzyme of the transsulfuration pathway leading to homocysteine synthesis was purified over 16,000-fold from spinach (Spinacia oleracea L.) leaf chloroplasts (soluble fraction). Enzyme activity was followed along the purification scheme by either a colorimetric method for
N Zhang et al.
International journal of plant sciences, 160(3), 511-519 (2001-09-07)
Lactuca sativa cv. Baijianye seedlings do not normally produce lateral roots, but removal of the root tip or application of auxin, especially indole-butyric acid, triggered the formation of lateral roots. Primordia initiated within 9 h and were fully developed after
Lasanthi P Jayathilaka et al.
Organic letters, 6(21), 3659-3662 (2004-10-08)
[reaction: see text] L-alpha-(1-Cyclobutenyl)glycine (1-Cbg) was targeted as a potentially translatable analogue of isoleucine and valine and as a useful building block for peptides. An enantioselective synthesis was executed in which the key step was diastereoselective addition of 1-cyclobutenylmagnesium bromide
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.