추천 제품
분석
≥98% (HPLC)
Quality Level
양식
solid
색상
yellow
solubility
DMSO: 0.39 mg/mL
ethanol: 0.4 mg/mL
0.1 M HCl: 0.68 mg/mL
methanol: 1 mg/mL
H2O: >10 mg/mL
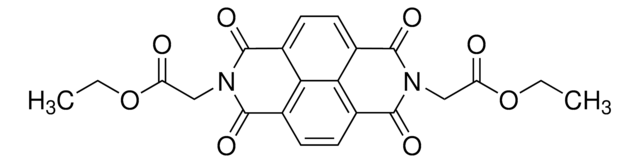
SMILES string
Cl[H].CC1=NN=C(c2ccc(N)cc2)c3cc4OCOc4cc3C1
InChI
1S/C17H15N3O2.ClH/c1-10-6-12-7-15-16(22-9-21-15)8-14(12)17(20-19-10)11-2-4-13(18)5-3-11;/h2-5,7-8H,6,9,18H2,1H3;1H
InChI key
RUBSCPARMVJNKX-UHFFFAOYSA-N
유전자 정보
human ... GRIA1(2890) , GRIA2(2891) , GRIA3(2892) , GRIA4(2893)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
GYKI 52466 hydrochloride has been used as an α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor selective antagonist in cell viability assays to evaluate the specificity of kainate (KA) effect on cerebellar granule cells (CGCs). It has also been used as an AMPA blocker to study the function of glutamate in neuroadapted sindbis virus (NSV) -induced motor neuron death.
생화학적/생리학적 작용
GYKI 52466 serves as an antagonist of several processes, mediated by glutamate.
Selective allosteric AMPA receptor antagonist; anticonvulsant; skeletal muscle relaxant.
특징 및 장점
This compound is a featured product for Neuroscience research. Click here to discover more featured Neuroscience products. Learn more about bioactive small molecules for other areas of research at sigma.com/discover-bsm.
This compound is featured on the Glutamate Receptors (Ion Channel Family) page of the Handbook of Receptor Classification and Signal Transduction. To browse other handbook pages, click here.
법적 정보
Sold under exclusive license from the Institute for Drug Research Ltd., Budapest, Hungary.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
53rd National Meeting of the Italian Society of Biochemistry and Molecular Biology (SIB) and National Meeting of Chemistry of Biological Systems ? Italian Chemical Society (SCI - Section CSB) null
T J Wilding et al.
Molecular pharmacology, 47(3), 582-587 (1995-03-01)
Whole-cell recordings were used to study the antagonism of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-preferring and kainate-preferring receptors by 2,3-benzodiazepines. Current through kainate-preferring receptors was recorded in rat dorsal root ganglion (DRG) neuron-s, whereas AMPA receptor current was measured in cultured neurons from
Adolfo E Talpalar et al.
Frontiers in neural circuits, 4 (2010-09-17)
Locomotion is a fundamental motor act that, to a large degree, is controlled by central pattern-generating (CPG) networks in the spinal cord. Glutamate is thought to be responsible for most of the excitatory input to and the excitatory activity within
S D Donevan et al.
Neuroscience, 87(3), 615-629 (1998-10-03)
Allosteric regulators of alpha-amino-3-hydroxy-5-methyl-4-isoxazole-propionate (AMPA) receptors include 2,3-benzodiazepines such as GYKI 52466 and GYKI 53655 and the chaotropic anion thiocyanate that inhibit, and benzothiadiazines such as cyclothiazide that potentiate AMPA receptor currents. Here we sought to determine whether the allosteric
S D Donevan et al.
Neuron, 10(1), 51-59 (1993-01-01)
In whole-cell voltage-clamp recordings from cultured rat hippocampal neurons, the 2,3-benzodiazepine GYKI 52466 was a potent antagonist of kainate- and AMPA-activated currents (IC50 values, 7.5 and 11 microM, respectively), but was inactive against N-methyl-D-aspartate (NMDA) or gamma-aminobutyric acid responses. The
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.