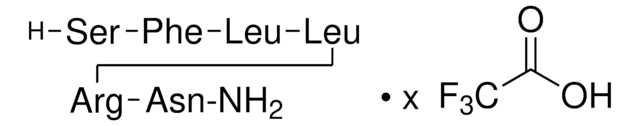F3681
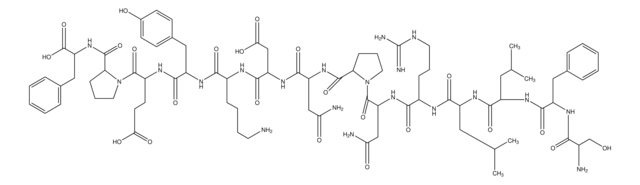
2-Furoyl-LIGRLO-amide trifluoroacetate salt
≥97% (HPLC)
동의어(들):
2-Fly, 2-Furoyl-Leu-Ile-Gly-Arg-Leu-Orn-NH2 trifluoroacetate salt, 2-f-LIGRLO-NH2, 2-fAP, 2fLI, f-LIGRLO-NH2
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C36H63N11O8 · xC2HF3O2
CAS Number:
Molecular Weight:
777.95 (free base basis)
MDL number:
UNSPSC 코드:
51111800
PubChem Substance ID:
NACRES:
NA.32
추천 제품
Quality Level
분석
≥97% (HPLC)
양식
lyophilized powder
색상
white
저장 온도
−20°C
SMILES string
Cc1cc(C)c2c(N)c(sc2n1)C(=O)NCc3ccc(Cl)cc3
InChI
1S/C17H16ClN3OS/c1-9-7-10(2)21-17-13(9)14(19)15(23-17)16(22)20-8-11-3-5-12(18)6-4-11/h3-7H,8,19H2,1-2H3,(H,20,22)
InChI key
FPRULFHDSFKYBV-UHFFFAOYSA-N
일반 설명
2-Furoyl-LIGRLO-amide trifluoroacetate salt is a peptide that acts as a proteinase-activated receptor-2 (PAR2) agonist, and contains a furoyl group addition at its N-terminal.
애플리케이션
2-Furoyl-LIGRLO-amide trifluoroacetate salt may be used as a protease-activated receptor 2 (PAR2) agonist in endothelial progenitor cells (EPCs) and in transient receptor potential cation channel subfamily V member (TRPV4)-transfected HEK293t cells.
생화학적/생리학적 작용
2-Furoyl-LIGRLO-amide is a potent and selective protease-activated receptor 2 (PAR2) agonist.
2-Furoyl-LIGRLO-amide is a potent and selective protease-activated receptor 2 (PAR2) agonist. PAR-2 activation is associated with increases in cAMP and intracellular Ca(2+). Immunoblot analysis revealed increases in phosphorylation of epidermal growth factor (EGF) receptor (EGFR) tyrosine kinase, Src, Pyk2, cRaf, and ERK1/2 in response to PAR-2 activation. 2-Furoyl-LIGRLO-amide is nearly 100-fold more potent than SLIGRL-NH2 (Cat. No. S9317). 2-Furoyl-LIGRLO-amide caused both an endothelium-dependent relaxation and an endothelium-independent contraction. It produced delayed (6 hours later) facilitation of capsaicin-evoked visceral nociception, an effect being much more potent than SLIGRL-NH2. Such effects were mimicked by i.col. trypsin. 2-f-LIGRL-NH2, coadministered repeatedly with caerulein six times in total, abolished the caerulein-evoked abdominal hyperalgesia/allodynia in WT, but not PAR2-KO, mice. Repeated doses of 2-f-LIGRL-NH2 moderately attenuated the severity of caerulein-induced pancreatitis in WT animals. It induced a similar dose-dependent increase in Ca2 levels in the presence and absence of b-arrestins.
2-Furoyl-LIGRLO-amide trifluoroacetate salt is a highly potent agonist of proteinase-activated receptor-2 (PAR2). In KNRK cells with high number of PAR2 receptors, this agonist causes elevation of intracellular Ca2+ levels. Studies on rat show that it causes the relaxation of aorta rings in an endothelium-dependent manner.
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
Morley D Hollenberg et al.
The Journal of pharmacology and experimental therapeutics, 326(2), 453-462 (2008-05-15)
The proteinase-activated receptor-2 (PAR2)-activating peptide with an N-terminal furoyl group modification, 2-furoyl-LIGRLO-NH2 (2fLI), was derivatized via its free ornithine amino group to yield [3H]propionyl-2fLI and Alexa Fluor 594-2fLI that were used as receptor probes for ligand binding assays and receptor
John J McGuire et al.
The Journal of pharmacology and experimental therapeutics, 309(3), 1124-1131 (2004-02-21)
A peptide corresponding to a proteinase-activated receptor 2 (PAR(2))-activating peptide with an N-terminal furoyl group modification, 2-furoyl-LIGRLO-NH(2), was assessed for PAR(2)-dependent and -independent biological activities. 2-Furoyl-LIGRLO-NH(2) was equally effective to and 10 to 25 times more potent than SLIGRLNH(2) for
Disrupting sensitization of TRPV4
Mack K and Fischer MJM
Neuroscience, 352, 1-8 (2017)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.