모든 사진(3)
About This Item
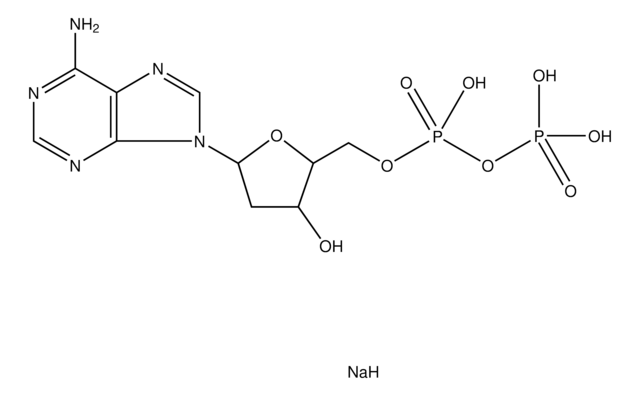
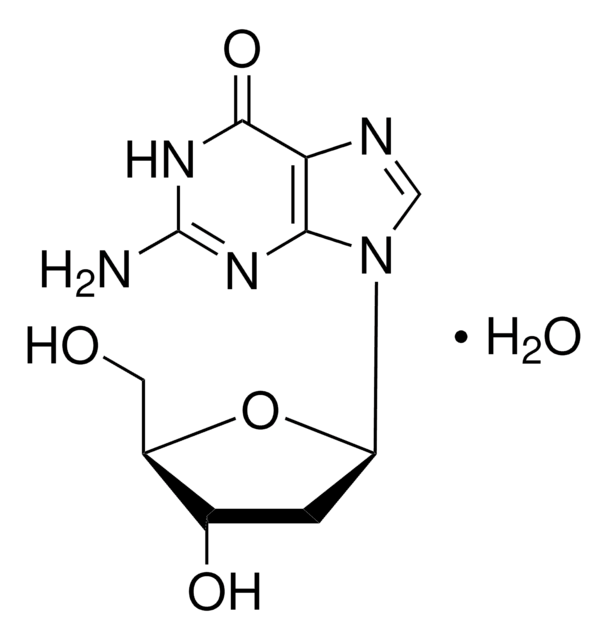
실험식(Hill 표기법):
C10H15N5O10P2 · xNa+
CAS Number:
Molecular Weight:
427.20 (free acid basis)
MDL number:
UNSPSC 코드:
41106305
PubChem Substance ID:
NACRES:
NA.51
추천 제품
분석
≥90% (HPLC)
양식
powder
solubility
water: 50 mg/mL, clear, colorless
저장 온도
−20°C
SMILES string
[Na].NC1=NC(=O)c2ncn(C3CC(O)C(COP(O)(=O)OP(O)(O)=O)O3)c2N1
InChI
1S/C10H15N5O10P2.Na.H/c11-10-13-8-7(9(17)14-10)12-3-15(8)6-1-4(16)5(24-6)2-23-27(21,22)25-26(18,19)20;;/h3-6,16H,1-2H2,(H,21,22)(H2,18,19,20)(H3,11,13,14,17);;
InChI key
VLWIICGGBFZYEZ-UHFFFAOYSA-N
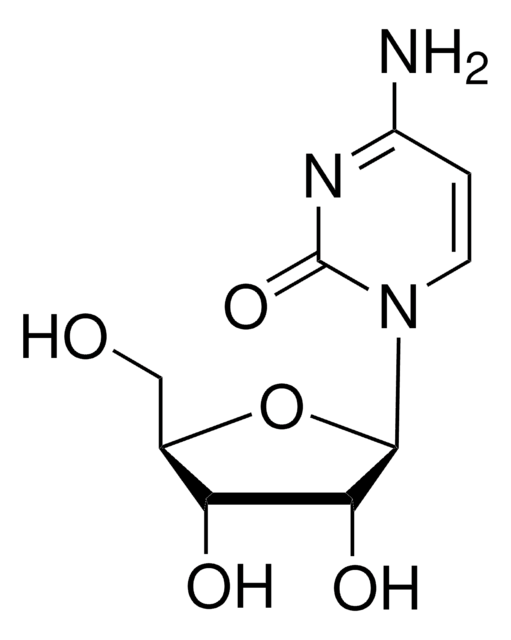
일반 설명
2′-Deoxyguanosine 5′-diphosphate (dGDP) is a nucleotide with guanine base, deoxyribose and two phosphate.
애플리케이션
2′-Deoxyguanosine 5′-diphosphate (dGDP) is used as a substrate of dGDP (nucleoside diphosphate) kinase (2.7.4.6) or pyruvate kinase to produce dGTP in support of DNA biosynthesis. dGDP is used as a substrate to study the kinetics of glycoprotein (gp) 1.7 expressed by bacteriophage T7.
2′-Deoxyguanosine 5′-diphosphate sodium salt has been used as a xanthosine 5′-monophosphate analog and xanthine phosphoribosyl transferase inhibitor in kinetic studies.
생화학적/생리학적 작용
2′-Deoxyguanosine 5′-diphosphate (dGDP) inhibits xanthine phosphoribosyl transferase (XPRT) and hypoxanthine-guanine phosphoribosyl transferase (HGPRT).
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
Ngoc Q Tran et al.
Molecular microbiology, 77(2), 492-504 (2010-05-26)
Gene 1.7 of bacteriophage T7 confers sensitivity of both phage T7 and its host Escherichia coli to dideoxythymidine (ddT). We have purified the product of gene 1.7, gp1.7. It exists in two forms of molecular weight 22,181 and 17,782. Only
Jie Bao et al.
Biotechnology and bioengineering, 89(4), 485-491 (2005-01-12)
The enzyme reaction mechanism and kinetics for biosyntheses of deoxyadenosine triphosphate (dATP) and deoxyguanosine triphosphate (dGTP) from the corresponding deoxyadenosine diphosphate (dADP) and deoxyguanosine diphosphate (dGDP) catalyzed by pyruvate kinase were studied. A kinetic model for this synthetic reaction was
Jiande Gu et al.
Nucleic acids research, 35(15), 5165-5172 (2007-07-31)
The 2'-deoxyguanosine-3',5'-diphosphate, 2'-deoxyadenosine-3',5'-diphosphate, 2'-deoxycytidine-3',5'-diphosphate and 2'-deoxythymidine-3',5'-diphosphate systems are the smallest units of a DNA single strand. Exploring these comprehensive subunits with reliable density functional methods enables one to approach reasonable predictions of the properties of DNA single strands. With these
Ngoc Q Tran et al.
Proceedings of the National Academy of Sciences of the United States of America, 105(27), 9373-9378 (2008-07-05)
Bacteriophage T7 DNA polymerase efficiently incorporates dideoxynucleotides into DNA, resulting in chain termination. Dideoxythymidine (ddT) present in the medium at levels not toxic to Escherichia coli inhibits phage T7. We isolated 95 T7 phage mutants that were resistant to ddT.
Bhumi Patel et al.
Biochimica et biophysica acta. Proteins and proteomics, 1866(3), 426-441 (2017-12-14)
Among all PRT enzymes of purine salvage pathway in Leishmania, XPRT (Xanthine phosphoribosyl transferase) is unique in its substrate specificity and their non-existence in human. It is an interesting protein not only for drug designing but also to understand the
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.