추천 제품
생물학적 소스
synthetic (organic)
Quality Level
분석
≥98% (TLC)
양식
powder
solubility
hot water: 19.60-20.40 mg/mL, clear, colorless
저장 온도
2-8°C
SMILES string
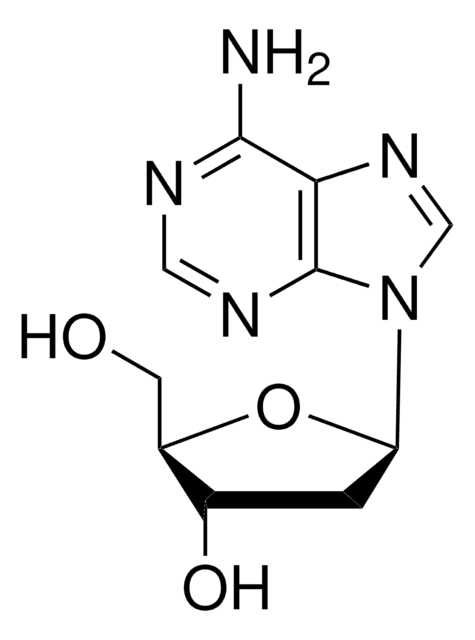
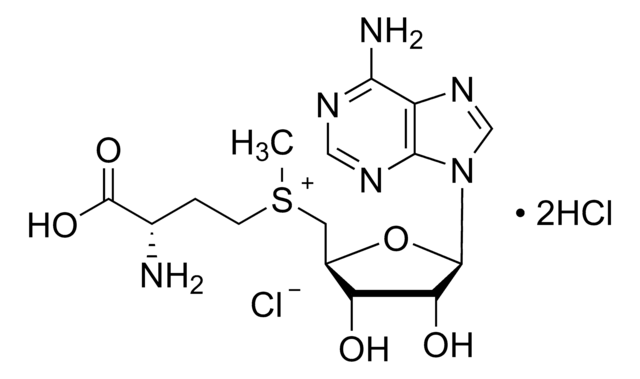
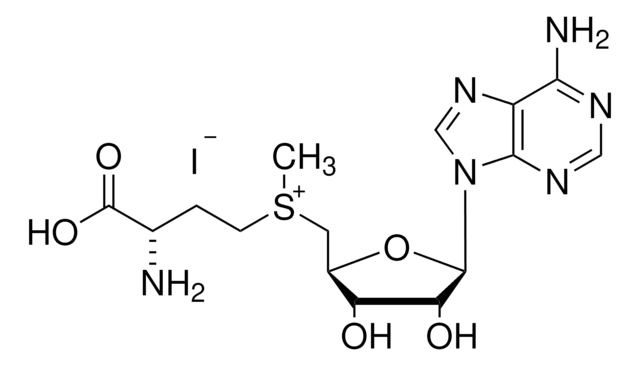
C[C@H]1O[C@H]([C@H](O)[C@@H]1O)n2cnc3c(N)ncnc23
InChI
1S/C10H13N5O3/c1-4-6(16)7(17)10(18-4)15-3-14-5-8(11)12-2-13-9(5)15/h2-4,6-7,10,16-17H,1H3,(H2,11,12,13)/t4-,6-,7-,10-/m1/s1
InChI key
XGYIMTFOTBMPFP-KQYNXXCUSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
5′-Deoxyadenosine has been used:
- as a standard in mass spectroscopy
- as an inhibitor for screening thymidine phosphorylase activity
- as a substrate in 5′-Deoxyadenosine deaminase (DadD) assay
생화학적/생리학적 작용
5′-Deoxyadenosine is a substrate for the enzyme methylthioadenosine/S-adenosylhomocysteine (MTA/SAH) nucleosidase in microbes. 5′-Deoxyadenosine is a byproduct of cleavage of S-adenosylmethionine (SAM). High levels of 5′-Deoxyadenosine inhibits SAM dependent enzymes. It also inhibits biotin synthase (BioB) and lipoyl synthase (LipA) enzymes.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
The nucleoside derivative 5?-O-trityl-inosine (KIN59) suppresses thymidine phosphorylase-triggered angiogenesis via a noncompetitive mechanism of action
Liekens S, et al.
Test, 279(28), 29598-29605 (2004)
Kenichi Yokoyama et al.
Biochemistry, 47(34), 8950-8960 (2008-08-05)
BtrN is a radical SAM ( S-adenosyl- l-methionine) enzyme that catalyzes the oxidation of 2-deoxy- scyllo-inosamine (DOIA) into 3-amino-2,3-dideoxy- scyllo-inosose (amino-DOI) during the biosynthesis of 2-deoxystreptamine (DOS) in the butirosin producer Bacillus circulans. Recently, we have shown that BtrN catalyzes
Charles J Walsby et al.
Inorganic chemistry, 44(4), 727-741 (2005-04-30)
Electron paramagnetic resonance (EPR), electron-nuclear double resonance (ENDOR), and Mössbauer spectroscopies and other physical methods have provided important new insights into the radical-SAM superfamily of proteins, which use iron-sulfur clusters and S-adenosylmethionine to initiate H atom abstraction reactions. This remarkable
Joseph T Jarrett
Current opinion in chemical biology, 7(2), 174-182 (2003-04-26)
Adenosylmethionine-dependent radical enzymes provide a novel mechanism for generating the highly oxidizing 5'-deoxyadenosyl radical in an anaerobic reducing environment. Recent studies suggest a unique covalent interaction between adenosylmethionine and a catalytic iron-sulfur cluster that may promote inner-sphere electron transfer to
Gunhild Layer et al.
Current opinion in chemical biology, 8(5), 468-476 (2004-09-29)
'Radical SAM' enzymes juxtapose a [4Fe-4S] cluster and S-adenosyl-l-methionine (SAM) to generate catalytic 5'-deoxyadenosyl radicals. The crystal structures of oxygen-independent coproporphyrinogen III oxidase HemN and biotin synthase reveal the positioning of both cofactors with respect to each other and relative
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.