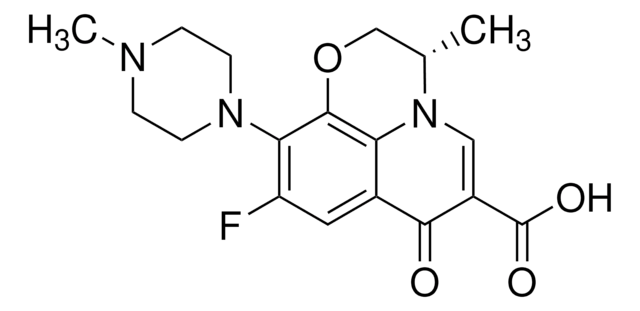
추천 제품
양식
powder or crystals
Quality Level
항생제 활성 스펙트럼
Gram-negative bacteria
동작 모드
DNA synthesis | interferes
enzyme | inhibits
SMILES string
CCN1N=C(C(O)=O)C(=O)c2cc3OCOc3cc12
InChI
1S/C12H10N2O5/c1-2-14-7-4-9-8(18-5-19-9)3-6(7)11(15)10(13-14)12(16)17/h3-4H,2,5H2,1H3,(H,16,17)
InChI key
VDUWPHTZYNWKRN-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Chemical structure: quinolone
애플리케이션
Cinoxacin was used to study the rat renal organic anion transporter 1 (OAT1). It is used to study fluoroquinolone-resistant Streptococcus pyogenes.
생화학적/생리학적 작용
Cinoxacin is a synthetic antimicrobial agent that interferes with DNA replication by inhibiting DNA gyrase and topoisomerase IV (topo IV) through tight DNA binding. The mechanism of action of cinoxacin is comparable to nalidixic acid. Cinoxacin is effective against Gram-negative bacteria and is often used to treat urinary tract infections caused by E. coli, Proteus mirabilis, Proteus vulgaris and Klebsiella sp.
기타 정보
Keep container tightly closed in a dry and well-ventilated place.
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
이미 열람한 고객
Lucija Peterlin-Masic et al.
Bioorganic & medicinal chemistry letters, 13(5), 789-794 (2003-03-06)
The design, synthesis and biological activity of non-covalent thrombin inhibitors incorporating 4,5,6,7-tetrahydroindazole, 2-methyl-4,5,6,7-tetrahydroindazole, 4,5,6,7-tetrahydroisoindole, 5,6,7,8-tetrahydroquinazoline and 5,6,7,8-tetrahydroquinazolin-2-amine as novel, partially saturated, heterobicyclic P(1)-arginine side-chain mimetics is described. The binding mode of the most potent candidate in the series co-crystallized with
D R Guay
Drug intelligence & clinical pharmacy, 16(12), 916-921 (1982-12-01)
Cinoxacin, a synthetic organic acid antibacterial agent, related structurally to nalidixic and oxolinic acid, has been approved for the treatment of initial and recurrent urinary tract infections (UTIs) caused by susceptible gram-negative microorganisms. The role of cinoxacin in the treatment
Kazuaki Arai et al.
The Journal of antimicrobial chemotherapy, 66(3), 494-498 (2010-12-22)
Streptococcus pyogenes causes various diseases in humans. While the prevalence of fluoroquinolone-resistant S. pyogenes isolates has been increasing since 2000 in the USA and Europe, it has remained very low in Japan. We isolated a fluoroquinolone-resistant S. pyogenes strain and
Franklin Vargas et al.
Journal of photochemistry and photobiology. B, Biology, 92(2), 83-90 (2008-06-20)
We have synthesized two naphthyl ester quinolone derivates and determined their ability to generate reactive oxygen species (ROS) such as (1)O(2), ()OH, H(2)O(2) upon photolysis with UV-A light. The ability of cinoxacin (1) and nalidixic acid (2), and their naphthyl
M Ruíz et al.
Journal of inorganic biochemistry, 69(4), 231-239 (1998-07-09)
Several cinoxacin (HCx) complexes with divalent metal ions have been prepared and characterized by spectroscopic techniques. The crystal structure of [Cd2(Cx)4(H2O)2].10H2O has been determined by X-ray diffraction. The complex is triclinic, space group P1 with unit-cell dimensions: a = 10.412(2)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.