A9043
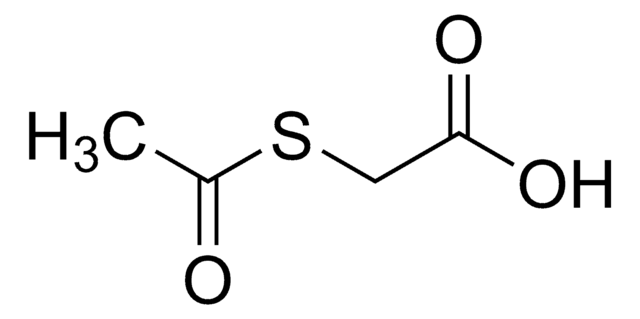
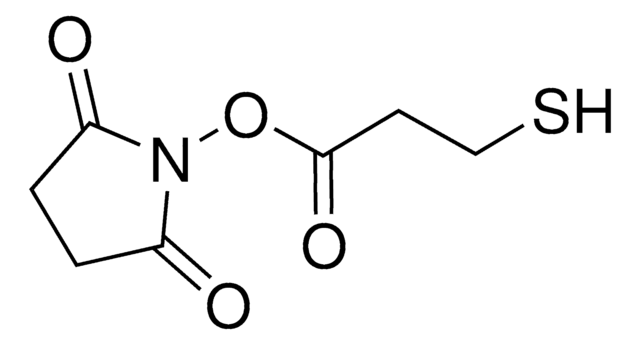
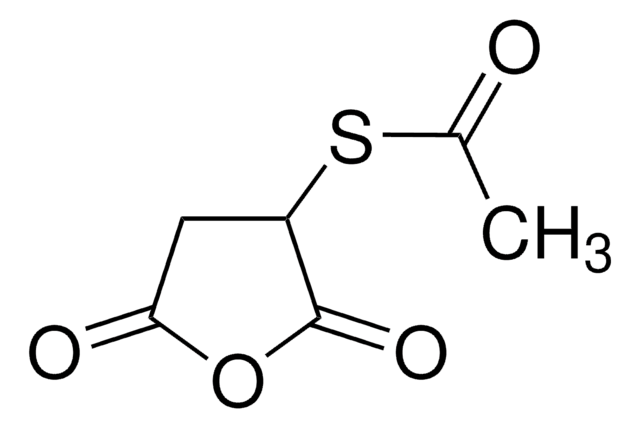
S-Acetylthioglycolic acid N-hydroxysuccinimide ester
≥95% (TLC), powder
동의어(들):
N-Succinimidyl (acetylthio)acetate, N-Succinimidyl S-acetylthioglycolate, S-Acetylthioglycolic acid NHS ester, SATA
로그인조직 및 계약 가격 보기
모든 사진(3)
About This Item
실험식(Hill 표기법):
C8H9NO5S
CAS Number:
Molecular Weight:
231.23
Beilstein:
7815491
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
애플리케이션
Thiolating reagent for primary amines; thiol can be deprotected under mild conditions. Typically, couples initially to a molecule containing primary amine by amide bond buffered at pH 7.5. Deprotection may be accomplished with 0.05 M hydroxylamine at neutral pH. Useful for preparation of enzyme immunoconjugates and hapten carrier molecule conjugates.
기타 정보
Note that the ester couples very efficiently to primary amines compared to other thiolating reagents. In addition, this reagent will neutralize original amine positive charge.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
가장 최신 버전 중 하나를 선택하세요:
시험 성적서(COA)
Lot/Batch Number
이미 열람한 고객
Jing-Tang Lin et al.
PloS one, 7(4), e36086-e36086 (2012-05-09)
To develop a fluorescent ruthenium complex for biosensing, we synthesized a novel sulfhydryl-reactive compound, 4-bromophenanthroline bis-2,2'-dipyridine Ruthenium bis (hexafluorophosphate). The synthesized Ru(II) complex was crosslinked with thiol-modified protein G to form a universal reagent for fluorescent immunoassays. The resulting Ru(II)-protein
R A Schwendener et al.
Biochimica et biophysica acta, 1026(1), 69-79 (1990-07-09)
The two coupling agents SPDP (N-succinimidyl-3-(2-pyridyldithio)propionate) and SATA (N-succinimidyl-S-acetylthioacetate) were compared in their efficiency and feasibility to couple monoclonal antibodies (Abs) via thioether linkage to liposomes functionalized by various lipophilic maleimide compounds like N-(3-maleimidopropionyl)-N2-palmitoyl-L-lysine methyl ester (MP-PL), N-(3-maleimidopropionyl)phosphatidylethanolamide (MP-PE), N6-(6-maleimidocaproyl)-N2-palmitoyl-L-lysine
Nejat Düzgüneş et al.
Methods in enzymology, 391, 351-373 (2005-02-22)
The intracellular activity of certain antiviral agents, including antisense oligonucleotides, acyclic nucleoside phosphonates, and protease inhibitors, is enhanced when they are delivered in liposome-encapsulated form. In this chapter we describe the preparation of pH-sensitive liposomes encapsulating antisense oligonucleotides, ribozymes, and
In Soo Shin et al.
Bioconjugate chemistry, 18(3), 821-828 (2007-03-23)
Sulfhydryl selective reactions were explored to conjugate oligomers of a peptidomimetic integrin alphavbeta3 antagonist, 4-[2-(3,4,5,6-tetrahydropyrimidine-2-ylamino)ethyloxy]benzoyl-2-(S)-aminoethylsulfonylamino-beta-alanine (IA) to monoclonal antibody (MoAb) to increase integrin alphavbeta3 receptor-binding avidity. To generate sulfhydryl groups, N-succinimidyl-S-acetylthioacetate (SATA) was conjugated to both MoAb and IA. Sulfhydryl
Ellen Verheyen et al.
Macromolecular bioscience, 10(12), 1517-1526 (2010-09-09)
An efficient strategy is reported to introduce methacrylamide groups on the lysine residues of a model protein (lysozyme) for immobilization and triggered release from a hydrogel network. A novel spacer unit was designed, containing a disulfide bond, such that the
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.