모든 사진(3)
About This Item
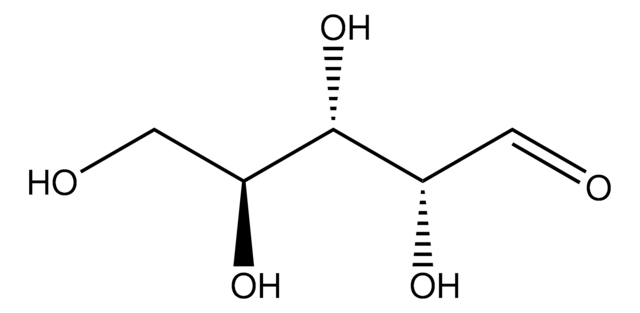
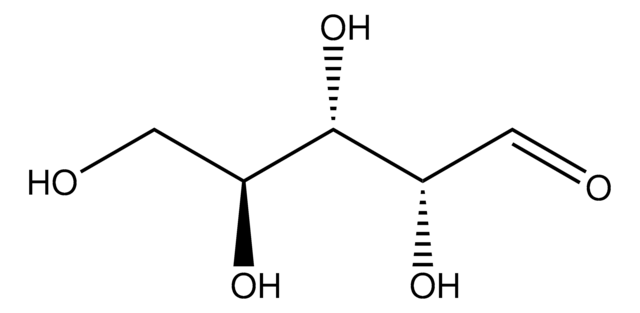
실험식(Hill 표기법):
C5H10O5
CAS Number:
Molecular Weight:
150.13
Beilstein:
1723079
EC Number:
MDL number:
UNSPSC 코드:
12352201
PubChem Substance ID:
NACRES:
NA.25
추천 제품
생물학적 소스
synthetic (organic)
Quality Level
분석
≥98% (GC)
양식
powder
광학 활성
[α]20/D -105 to -103 °, c = 4% (w/v) in water
기술
gas chromatography (GC): suitable
색상
white to off-white
mp
162-164 °C (lit.)
solubility
water: 50 mg/mL, clear, colorless to faintly yellow
저장 온도
room temp
SMILES string
O[C@@H]1COC(O)[C@@H](O)[C@@H]1O
InChI
1S/C5H10O5/c6-2-1-10-5(9)4(8)3(2)7/h2-9H,1H2/t2-,3-,4+,5?/m1/s1
InChI key
SRBFZHDQGSBBOR-ZRMNMSDTSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
D-(−)-Arabinose is a D-ribose analogue present in sugar polymers such as the arabinogalatans of mycobacterial cell walls and plant tissues. D-Arabinose is a substrate used to identify, differentiate and characterize arabinose isomerase(s) that have commercial value in the production of tagatose, a low calorie sweetener.
생화학적/생리학적 작용
D-Arabinose is a reducing sugar. It is a pentose analog of D-ribose that is a constituent of mycobacterial cell wall arabinogalactans. It is also a substrate for D-erythroascorbic acid synthesis in yeast.
기타 정보
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
Zhe Yang et al.
Proceedings of the National Academy of Sciences of the United States of America, 112(16), 5219-5224 (2015-04-08)
Starved animals often exhibit elevated locomotion, which has been speculated to partly resemble foraging behavior and facilitate food acquisition and energy intake. Despite its importance, the neural mechanism underlying this behavior remains unknown in any species. In this study we
Derek Thomson et al.
Pulmonary pharmacology & therapeutics, 40, 69-79 (2016-05-26)
It is now recognized that certain polysaccharides can exhibit anti-inflammatory activity, including the glycosaminoglycan (GAG) heparin that is widely used as an anti-coagulant drug. However, it would be desirable to identify molecules that retain the anti-inflammatory actions of heparin, but
Nobuhiro Yamagata et al.
Proceedings of the National Academy of Sciences of the United States of America, 112(2), 578-583 (2014-12-31)
Drosophila melanogaster can acquire a stable appetitive olfactory memory when the presentation of a sugar reward and an odor are paired. However, the neuronal mechanisms by which a single training induces long-term memory are poorly understood. Here we show that
W Joost Wiersinga et al.
Emerging infectious diseases, 21(1), 40-47 (2014-12-23)
Burkholderia pseudomallei, an environmental gram-negative bacillus, is the causative agent of melioidosis and a bio-threat agent. Reports of B. pseudomallei isolation from soil and animals in East and West Africa suggest that melioidosis might be more widely distributed than previously
Arie Gruzman et al.
Journal of medicinal chemistry, 51(24), 8096-8108 (2008-12-04)
Type 2 diabetes mellitus has reached epidemic proportions; therefore, the search for novel antihyperglycemic drugs is intense. We have discovered that D-xylose increases the rate of glucose transport in a non-insulin-dependent manner in rat and human myotubes in vitro. Due
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.