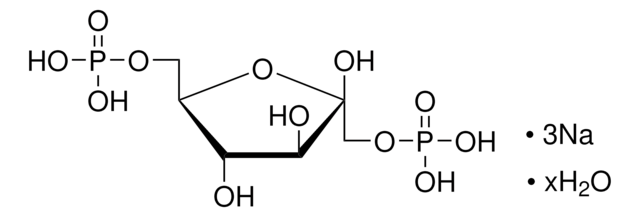
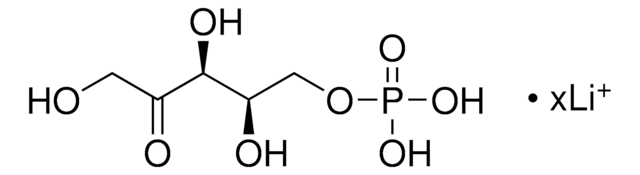
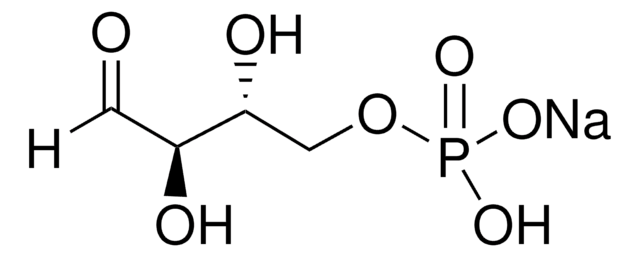
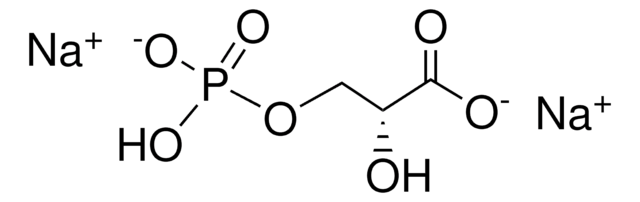
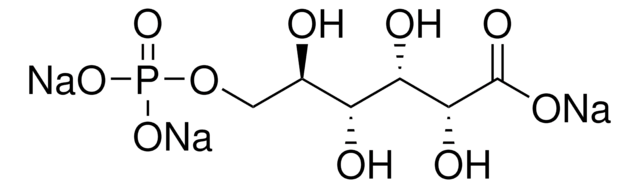
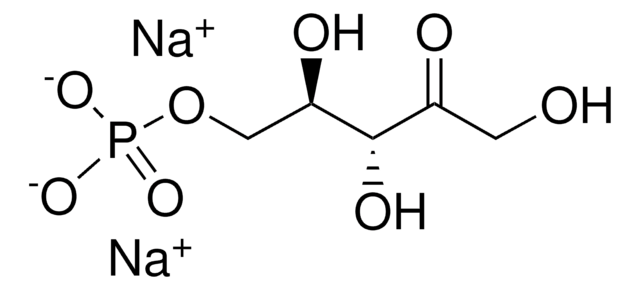
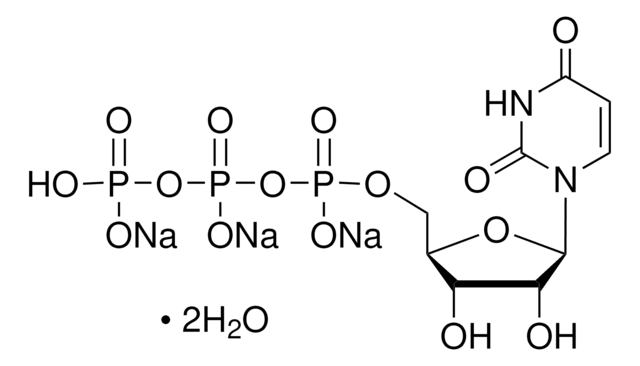
추천 제품
Quality Level
분석
≥90% (TLC)
양식
powder
색상
white to light yellow
저장 온도
−20°C
InChI
1S/C7H15O10P/c8-1-3(9)5(11)7(13)6(12)4(10)2-17-18(14,15)16/h4-8,10-13H,1-2H2,(H2,14,15,16)/t4-,5-,6-,7+/m1/s1
InChI key
JDTUMPKOJBQPKX-GBNDHIKLSA-N
생화학적/생리학적 작용
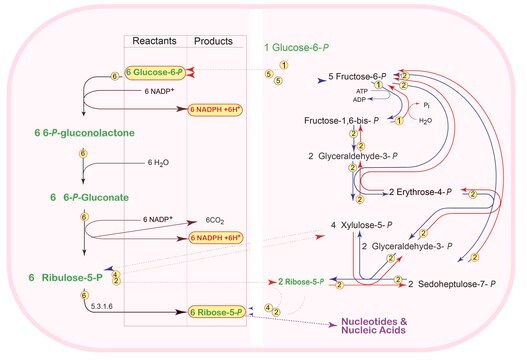
D-Sedoheptulose 7-phosphate is a metabolite in a number of pathways, e.g. an intermediate in the pentose phosphate pathway, and the carbon fixation in photosynthetic organisms.
D-Sedoheptulose is a pentose phosphate pathway (PPP) intermediate. D-Sedoheptulose-7-phosphate contributes to the generation of NADPH and the formation of ribose residues of nucleotide synthesis. Sedoheptulose-7-phosphate levels may be increased in individuals with defects in transaldolase (TALSDO1).
포장
Bottomless glass bottle. Contents are inside inserted fused cone.
기타 정보
To gain a comprehensive understanding of our extensive range of Monosaccharides for your research, we encourage you to visit our Carbohydrates Category page.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
L Eidels et al.
Proceedings of the National Academy of Sciences of the United States of America, 68(8), 1673-1677 (1971-08-01)
Genetic and biochemical evidence that sedoheptulose-7-phosphate is an obligatory precursor of the L-glycero-D-mannoheptose residues of the lipopolysaccharide of Salmonella was obtained by isolation and characterization of transketolase-negative mutants of Salmonella typhimurium. These mutants, which are defective in synthesis of sedoheptulose-7-phosphate
Sedoheptulose kinase regulates cellular carbohydrate metabolism by sedoheptulose 7-phosphate supply.
Csörsz Nagy et al.
Biochemical Society transactions, 41(2), 674-680 (2013-03-22)
Dynamic carbon re-routing between catabolic and anabolic metabolism is an essential element of cellular transformation associated with tumour formation and immune cell activation. Such bioenergetic adaptations are important for cellular function and therefore require tight control. Carbohydrate phosphorylation has been
John F Williams et al.
Photosynthesis research, 90(2), 125-148 (2006-12-13)
14C-Labelled octulose phosphates were formed during photosynthetic 14CO2 fixation and were measured in spinach leaves and chloroplasts. Because mono- and bisphosphates of D: -glycero- D: -ido-octulose are the active 8-carbon ketosugar intermediates of the L-type pentose pathway, it was proposed
Biosynthesis of 4-hydroxy-2(or 5)-ethyl-5(or 2)-methyl-3(2H)-furanone by yeasts.
Sasaki, M., et al.
Journal of Agricultural and Food Chemistry, 39, 934-938 (1991)
Mirjam M C Wamelink et al.
Journal of chromatography. B, Analytical technologies in the biomedical and life sciences, 823(1), 18-25 (2005-08-02)
We describe a liquid chromatography tandem mass spectrometry (LC-MS/MS) method to quantify pentose phosphate pathway intermediates (triose-3-phosphates, tetrose-4-phosphate, pentose-5-phosphate, pentulose-5-phosphates, hexose-6-phosphates and sedoheptulose-7-phosphate (sed-7P)) in bloodspots, fibroblasts and lymphoblasts. Liquid chromatography was performed using an ion pair loaded C(18) HPLC
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.