47614
Fluram
BioReagent, suitable for fluorescence, ≥99.0% (UV)
동의어(들):
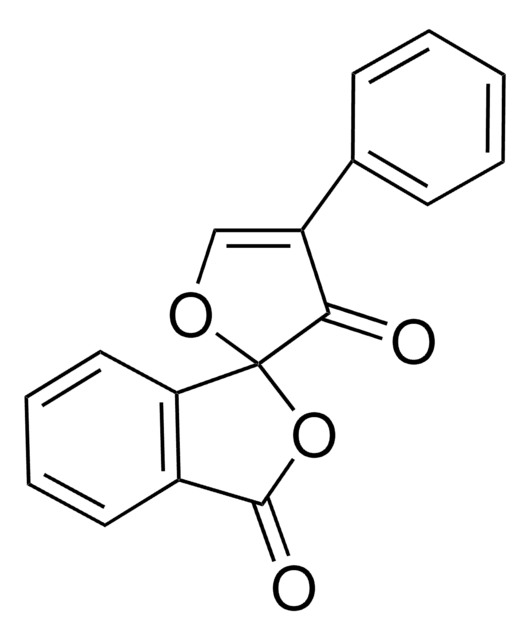
Fluorescamine, 4-Phenylspiro-[furan-2(3H),1-phthalan]-3,3′-dione
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C17H10O4
CAS Number:
Molecular Weight:
278.26
Beilstein:
921143
EC Number:
MDL number:
UNSPSC 코드:
12352200
PubChem Substance ID:
추천 제품
제품 라인
BioReagent
분석
≥99.0% (UV)
mp
153-157 °C (lit.)
153-157 °C
solubility
acetonitrile: soluble
ethanol: soluble
형광
λex 234 nm
λex 390 nm; λem 480 nm in 0.5 M borate pH 8.5 (after derivatization with L-leucine)
적합성
suitable for fluorescence
SMILES string
O=C1OC2(OC=C(C2=O)c3ccccc3)c4ccccc14
InChI
1S/C17H10O4/c18-15-13(11-6-2-1-3-7-11)10-20-17(15)14-9-5-4-8-12(14)16(19)21-17/h1-10H
InChI key
ZFKJVJIDPQDDFY-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
Fluram (fluorescamine) is a non-fluorescent reagent that reacts readily under mild conditions with primary amines in amino acids and peptides and other molecules to form stable, highly fluorescent compounds. Fluorescamine is useful for the fluorometric assay of amino acids, protein, and proteolytic enzymes and as a pre-column derivatization reagent. It effectively blocks newly generated amino termini in protein sequence analyses.
Non-fluorescent reagent that reacts readily under mild conditions with primary amines in amino acids and peptides to form stable, highly fluorescent compounds. Low background due to hydrolysis. Useful for the fluorometric assay of amino acids, protein, and proteolytic enzymes. Effectively blocks newly generated amino termini in protein sequence analyses.
포장
Bottomless glass bottle. Contents are inside inserted fused cone.
법적 정보
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Gloves, type N95 (US)
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
José Pedro Castro et al.
Redox biology, 21, 101108-101108 (2019-01-21)
Aging is accompanied by the accumulation of oxidized proteins. To remove them, cells employ the proteasomal and autophagy-lysosomal systems; however, if the clearance rate is inferior to its formation, protein aggregates form as a hallmark of proteostasis loss. In cells
Wen-Hsien Tsai et al.
Journal of chromatography. A, 1217(49), 7812-7815 (2010-11-04)
A simple sugaring-out assisted liquid-liquid extraction method combined with high-performance liquid-chromatography with fluorescence detection (HPLC-FL) was developed for the extraction and determination of sulfonamides in honey. Sample preparation consisted of acid hydrolysis to release sugar-bound sulfonamides. After derivatization with fluorescamine
Manjeet Deshmukh et al.
Biomaterials, 31(26), 6675-6684 (2010-06-22)
Two vinyl sulfone functionalized crosslinkers were developed for the purpose of preparing degradable poly(ethylene glycol) (PEG) hydrogels (EMXL and GABA-EMXL hydrogels). A self-elimination degradation mechanism in which an N-terminal residue of a glutamine is converted to pyroglutamic acid with subsequent
Laura Contreras-Ruiz et al.
Cornea, 29(5), 550-558 (2010-03-26)
Hyaluronic acid-chitosan nanoparticles (HA-CS NPs) have the potential to serve as a reliable drug delivery system to topically treat ocular surface disorders. We evaluated the in vivo uptake by ocular structures, the acute tolerance, and possible alterations of tear film
Grégoire Danger et al.
Electrophoresis, 29(19), 4036-4044 (2008-10-30)
The first results of chiral separations with the gradient elution isotachophoresis method are presented. As previously described, citrate is used in the run buffer as the leading ion and borate in the sample buffer as the terminating ion. Modulation of
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.