추천 제품
vapor density
3.9 (vs air)
Quality Level
vapor pressure
97.5 mmHg ( 20 °C)
제품 라인
ReagentPlus®
분석
99%
형태
liquid
불순물
≤0.05% water
refractive index
n20/D 1.3 (lit.)
pH
1 (10 g/L)
bp
72.4 °C (lit.)
mp
−15.4 °C (lit.)
solubility
ethanol: soluble 0.33 mL/mL
density
1.489 g/mL at 20 °C (lit.)
SMILES string
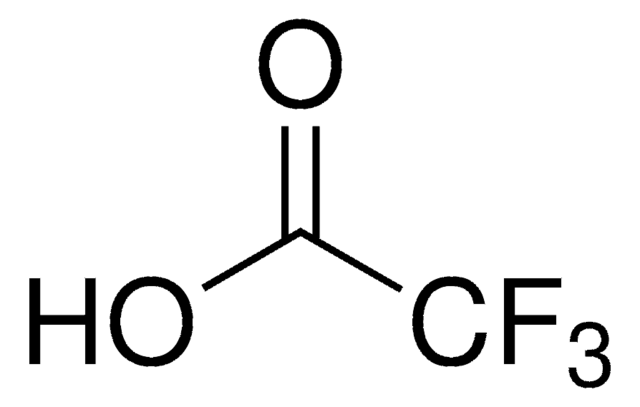
OC(C(F)(F)F)=O
InChI
1S/C2HF3O2/c3-2(4,5)1(6)7/h(H,6,7)
InChI key
DTQVDTLACAAQTR-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
애플리케이션
- For the cleavage of nitrogen and oxygen protecting groups such as N-Boc, N-benzyloxymethyl, benzyl ether, p-methoxybenzyl ether, t-butyl ether, t-butyloxymethyl ether, triphenylmethyl ether, and dimethyl acetals.
- In the Baeyer–Villiger oxidation reactions in combination with sodium percarbonate.,·
- For the C-H trifluoromethylation of arenes.
TFA can also be used as:
- A solvent in atom transfer cyclization reactions and polymer processes.
- A catalyst in the synthesis of ε-caprolactam via Beckmann rearrangement of cyclohexanone oxime in aprotic solvents.
포장
법적 정보
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Inhalation - Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1A
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 2
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
프로토콜
Fmoc resin cleavage and deprotection follows the difficult task of detaching the peptide from the resin support and removing all the side-chain protecting groups of the amino acid residues to yield the desired peptide.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.