E27408
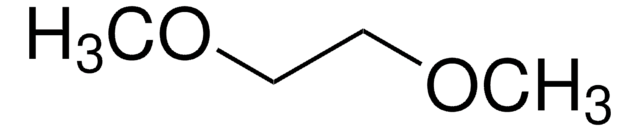
1,2-Dimethoxyethane
ReagentPlus®, ≥99%, inhibitor-free
동의어(들):
mono-Glyme, Dimethylglycol, Ethylene glycol dimethyl ether, Monoglyme
로그인조직 및 계약 가격 보기
모든 사진(4)
About This Item
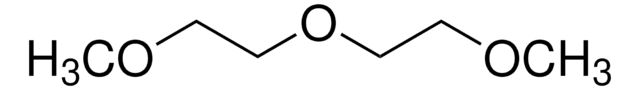
Linear Formula:
CH3OCH2CH2OCH3
CAS Number:
Molecular Weight:
90.12
Beilstein:
1209237
EC Number:
MDL number:
UNSPSC 코드:
12352112
PubChem Substance ID:
NACRES:
NA.21
분석:
≥99%
bp:
85 °C (lit.)
vapor pressure:
48 mmHg ( 20 °C)
추천 제품
vapor density
3.1 (20 °C, vs air)
Quality Level
vapor pressure
48 mmHg ( 20 °C)
제품 라인
ReagentPlus®
분석
≥99%
양식
liquid
autoignition temp.
396 °F
expl. lim.
10.4 %
불순물
≤0.5% (water)
refractive index
n20/D 1.379 (lit.)
pH
7
bp
85 °C (lit.)
mp
−58 °C (lit.)
density
0.867 g/mL at 25 °C (lit.)
SMILES string
COCCOC
InChI
1S/C4H10O2/c1-5-3-4-6-2/h3-4H2,1-2H3
InChI key
XTHFKEDIFFGKHM-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
1,2-Dimethoxyethane (DME) is an inert aprotic polar solvent for electrolytes of lithium batteries, polysilicones, oligo- and polysaccharides.
애플리케이션
1,2-Dimethoxyethane can be used as a solvent to synthesize:
- Oxazoles from α-acylamino ketones via Robinson-Gabriel reaction.
- 5-Arylhistidines via Suzuki-Miyaura cross-coupling between 5-bromohistidine and arylboronic acids.
- Aryl thioethers via Pd-catalyzed thioetherification of aryl halides with aliphatic and aromatic thiols.
법적 정보
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Inhalation - Flam. Liq. 2 - Repr. 1B - Skin Irrit. 2
보충제 위험성
Storage Class Code
3 - Flammable liquids
WGK
WGK 1
Flash Point (°F)
41.0 °F - closed cup
Flash Point (°C)
5 °C - closed cup
이미 열람한 고객
Tetrahedron Letters, 34, 4591-4591 (1993)
Organometallics, 13, 1498-1498 (1994)
Inorganic Chemistry, 33, 1685-1685 (1994)
Shaokun Tang et al.
Bioresource technology, 129, 667-671 (2013-01-10)
Glymes (i.e. glycol diethers) were explored as alternative benign solvents for enzymatic reactions, specifically the lipase-catalyzed transesterification. Long-chain glymes were found highly compatible with immobilized Candida antarctica lipase B (iCALB), leading to higher enzyme activities and stabilities than t-butanol and
Abhishek K Jha et al.
The Journal of chemical physics, 128(3), 034501-034501 (2008-01-22)
The physical content of and, in particular, the nonlinear contributions from the Langevin-Debye model are illustrated using two applications. First, we provide an improvement in the Langevin-Debye model currently used in some implicit solvent models for computer simulations of solvation
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.