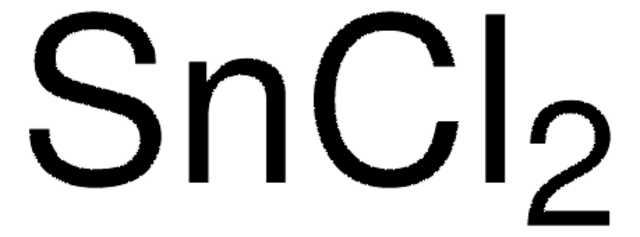모든 사진(5)
About This Item
Linear Formula:
SnCl2 · 2H2O
CAS Number:
Molecular Weight:
225.65
EC Number:
MDL number:
UNSPSC 코드:
12352302
PubChem Substance ID:
NACRES:
NA.21
분석:
98%
98.0-103.0% (ACS specification)
98.0-103.0% (ACS specification)
Grade:
ACS reagent
양식:
powder or crystals
solubility:
hydrochloric acid: passes test
water: soluble
water: soluble
추천 제품
Grade
ACS reagent
Quality Level
분석
98%
98.0-103.0% (ACS specification)
양식
powder or crystals
반응 적합성
reagent type: catalyst
core: tin
bp
652 °C (lit.)
mp
37-38 °C (dec.) (lit.)
solubility
hydrochloric acid: passes test
water: soluble
음이온 미량물
sulfate (SO42-): passes test (lim. ~0.003%)
양이온 미량물
Ca: ≤0.005%
Fe: ≤0.003%
K: ≤0.005%
Na: ≤0.01%
Pb: ≤0.01%
SMILES string
O.O.Cl[SnH2]Cl
InChI
1S/2ClH.2H2O.Sn/h2*1H;2*1H2;/q;;;;+2/p-2
InChI key
FWPIDFUJEMBDLS-UHFFFAOYSA-L
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Crystals of SnCl2.2H2O exhibit exhibit monoclinic crystal system and space group P21/c.
애플리케이션
Tin(II) chloride dihydrate (SnCl2.2H2O)may be used in the preparation of indoles. It may be used in the transformation of conjugated dioxolones, conjugated and non-conjugated acetals (dimethoxy and diethoxy acetals) to aldehydes.
It plays the role of a catalyst in the synthesis of 3-aminoimidazo[1,2-a]pyridines. It can be used as precursor for the synthesis of tin dioxide (SnO2) nanostructures by thermal degradation at temperature between 400-700 under controlled conditions.
It plays the role of a catalyst in the synthesis of 3-aminoimidazo[1,2-a]pyridines. It can be used as precursor for the synthesis of tin dioxide (SnO2) nanostructures by thermal degradation at temperature between 400-700 under controlled conditions.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Met. Corr. 1 - Skin Corr. 1B - Skin Sens. 1 - STOT RE 2 Oral - STOT SE 3
표적 기관
Cardio-vascular system, Respiratory system
Storage Class Code
8B - Non-combustible corrosive hazardous materials
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
이미 열람한 고객
An X-Ray Redetermination of the Crystal Structure of Tin (II) Chloride Dihydrate.
Kiriyama H, et al.
Bulletin of the Chemical Society of Japan, 46(5), 1389-1395 (1973)
Tin (II) chloride dihydrate: A mild and efficient reagent for cleaving acetals.
Ford KL and Roskamp EJ.
Tetrahedron Letters, 33(9), 1135-1138 (1992)
Tin (II) Chloride Dihydrate Catalyzed Groebke Condensation: An Efficient Protocol for the Synthesis of 3-Aminoimidazo[1,2-a]pyridines.
Shaabani A, et al.
Chin. J. Chem., 27(2), 369-371 (2009)
Ruthenium-catalysed synthesis of indoles from anilines and trialkanolamines in the presence of tin (II) chloride dihydrate.
Cho CS.
Chemical Communications (Cambridge, England), 9, 995-996 (1998)
Optical properties of SnO2 nanostructures prepared via one-step thermal decomposition of tin (II) chloride dihydrate.
Al-Gaashani R, et al.
Materials Science and Engineering, B, 177(6), 462-470 (2012)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.