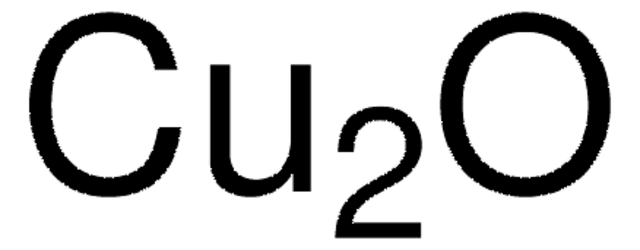추천 제품
Quality Level
분석
97%
형태
powder
포함
stabilizer
입자 크기
≤7 μm
density
6 g/mL at 25 °C (lit.)
SMILES string
[Cu]O[Cu]
InChI
1S/2Cu.O
InChI key
BERDEBHAJNAUOM-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
관련 카테고리
일반 설명
Copper(I)oxide is a weak base that can be used in nucleophilic substitution, decarboxylation, cyclocondensation, and 1,1-addition reactions. It can also be used to form carbenoid intermediates from diazo compounds and radicals from arenediazonium salts.
애플리케이션
Copper(I) oxide can be used as a catalyst to synthesize:
- Ynones via solvent-free Sonogashira reaction.
- N-Heteroaryl derivatives via cross-coupling of nitrogen heterocycles with halopyridines.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
시험 성적서(COA)
제품의 로트/배치 번호를 입력하여 시험 성적서(COA)을 검색하십시오. 로트 및 배치 번호는 제품 라벨에 있는 ‘로트’ 또는 ‘배치’라는 용어 뒤에서 찾을 수 있습니다.
이미 열람한 고객
Dmytro S Kozak et al.
Scientific reports, 6, 21178-21178 (2016-02-18)
Electrolytic processes are widely used to synthesize different nanomaterials and it does not depend on what kind of the method has been applied (wet-chemistry, sonochemistry, plasma chemistry, electrolysis and so on). Generally, the reactions in the electrolyte are considered to
Copper (I) Oxide-Mediated Cyclization of o-Haloaryl N-Tosylhydrazones: Efficient Synthesis of Indazoles.
Tang M, et al.
Advanced Synthesis & Catalysis (2016)
Copper (I) Oxide
Tuckmantel W
e-EROS Encyclopedia of Reagents for Organic Synthesis (2001)
Electrochemical deposition of copper (I) oxide films.
Golden TD, et al.
Chemistry of Materials, 8(10), 2499-2504 (1996)
A highly efficient nano-sized Cu2O/SiO2 egg-shell catalyst for C?C coupling reactions
Kim S, et al.
Royal Society of Chemistry Advances, 8, 6200-6205 (2018)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.