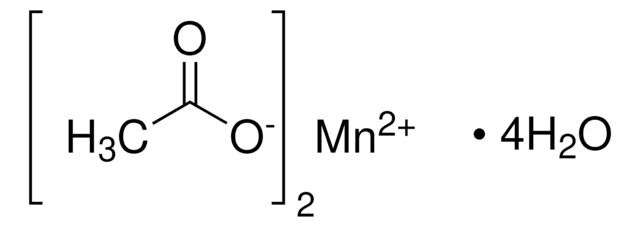
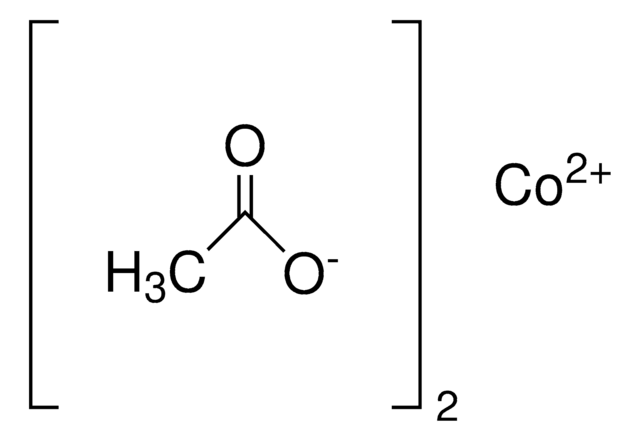
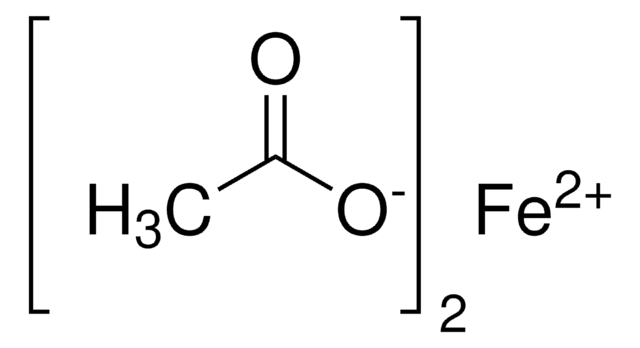
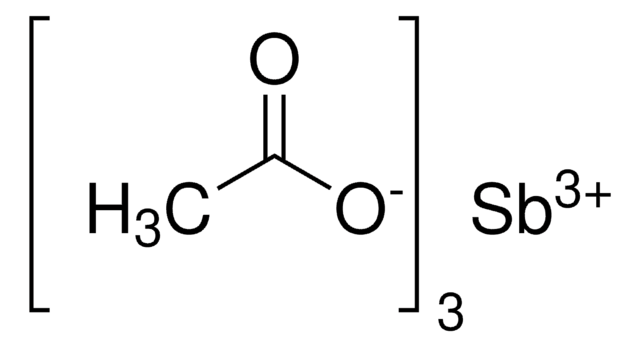
추천 제품
Grade
reagent grade
양식
powder or crystals
농도
23.0-25.0% Co (EDTA titration)
SMILES string
[H]O[H].[H]O[H].[H]O[H].[H]O[H].CC(=O)O[Co]OC(C)=O
InChI
1S/2C2H4O2.Co.4H2O/c2*1-2(3)4;;;;;/h2*1H3,(H,3,4);;4*1H2/q;;+2;;;;/p-2
InChI key
ZBYYWKJVSFHYJL-UHFFFAOYSA-L
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Cobalt(II) acetate tetrahydrate, commonly used as bleach and drying agent, can be produced by reacting cobalt (II) carbonate and acetic acid. It undergoes decomposition on heating up to 500°C under non-isothermal conditions. Its crystals exhibit monoclinic crystal system having space group P21/c. Cobalt(II) acetate tetrahydrate undergoes hydrothermal reaction with 1,3,5-benzenetricarboxylic acid (BTCH3) to afford Co3(BTC)2.12H2O.
애플리케이션
Cobalt(II) acetate tetrahydrate may be used as a cobalt source in the synthesis of cobalt nanoparticles and cobalt oxide thin films. It may also be used to prepare cobalt(II)-aminophenyltetrazolate coordination polymer and tricobalt complexes with mixed μ-acetato and μ-pyrazolato ligands. Cobalt(II) acetate tetrahydrate enhances the reaction rate of oxidation of 1,4-dihydropyridines using hydrogen peroxide to the corresponding pyridine derivatives.
Cobalt(II) acetate tetrahydrate may be used in the preparation of [Co3(OH)2(H2O)2(aptet)4] (aptet = 4-aminophenyltetrazolate) and new tetradentate cobalt(II)-Schiff base complex.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Carc. 1B Inhalation - Eye Irrit. 2 - Muta. 2 - Repr. 1B - Resp. Sens. 1 - Skin Sens. 1
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
이미 열람한 고객
Synthesis, characterization, and electrochemical behavior of a cobalt (II) salen-like complex.
Ourari A, et al.
Polyhedron, 97, 197-201 (2015)
Aromatization of Hantzsch 1, 4-dihydropyridines by hydrogen peroxide in the presence of cobalt (II) acetate.
Hashemi MM, et al.
Monatshefte fur Chemie / Chemical Monthly, 134(1), 107-110 (2003)
Cobalt nanoparticles synthesis from Co(CH3COO)2 by thermal decomposition.
Shao H, et al.
Journal of magnetism and magnetic materials, 304(1), e28-e30 (2006)
The non-isothermal decomposition of cobalt acetate tetrahydrate: a kinetic and thermodynamic study.
Mohamed M, et al.
Journal of Thermal Analysis and Calorimetry, 1(2-3), 387-404 (1994)
Chemical accuracy and precision in Rietveld analysis: The crystal structure of cobalt (II) acetate tetrahydrate.
Kaduk JA and Walt Partenheimer.
Powder Diffraction, 12(01), 27-39 (1997)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.