Y0001522
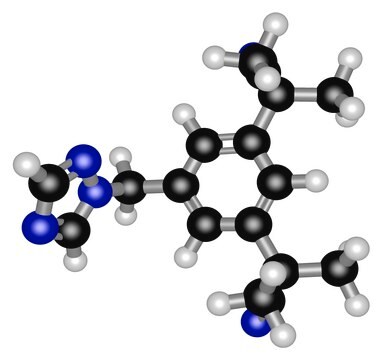
Anastrozole
European Pharmacopoeia (EP) Reference Standard
동의어(들):
2;2"-[5-(1H-1;2;4-Triazol-1-ylmethyl)-1, 3-Phenylene]bis(2-methyl-propiononitrile, Arimidex, ICI-D1033, ZD1033, a,a,a′,a′-Tetramethyl-5-(1H-1,2,4-triazol-1-ylmethyl)-1,3-benzenediacetonitrile
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
추천 제품
Grade
pharmaceutical primary standard
API family
anastrozole
제조업체/상표
EDQM
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-8°C
InChI
1S/C17H19N5/c1-16(2,9-18)14-5-13(8-22-12-20-11-21-22)6-15(7-14)17(3,4)10-19/h5-7,11-12H,8H2,1-4H3
InChI key
YBBLVLTVTVSKRW-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Anastrozole EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
포장
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
기타 정보
Sales restrictions may apply.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Repr. 1B
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
Jennifer L Lycette et al.
Breast cancer research and treatment, 99(3), 249-255 (2006-06-06)
Hormonal therapy is the mainstay of adjuvant treatment for women with early-stage estrogen receptor-positive breast cancer. Recently, the aromatase inhibitors have moved to the forefront of adjuvant hormonal therapy, however, the adverse effects of these agents are not yet fully
Mark Sanford et al.
Drugs, 68(9), 1319-1340 (2008-06-13)
Anastrozole (Arimidex) is an aromatase inhibitor approved in the EU, the US and in other countries worldwide for use as an adjuvant treatment in postmenopausal women with early-stage, hormone receptor-positive breast cancer. It is also approved in the EU and
Robert W Carlson et al.
Breast cancer research and treatment, 80 Suppl 1, S19-S26 (2003-10-11)
The use of adjuvant endocrine therapy in the treatment of hormone receptor-positive, early breast cancer has become important in both pre- and postmenopausal women. Tamoxifen has been the principal adjuvant hormonal therapy in pre- and postmenopausal women with hormone receptor-positive
Raimund Jakesz
Expert opinion on drug metabolism & toxicology, 2(2), 301-312 (2006-07-27)
Anastrozole is a nonsteroidal, third-generation aromatase inhibitor, which is heralded as an effective alternative endocrine therapy to tamoxifen in postmenopausal women with hormone-responsive breast cancer. Anastrozole has a high affinity for aromatase, a CYP enzyme involved in estrogen synthesis, and
K Mokbel
Current medical research and opinion, 19(8), 683-688 (2003-12-23)
This commentary article provides an overview of recent clinical research trials involving anastrozole and its evolving role in the management of breast cancer. Anti-aromatase agents inhibit the cytochrome P-450 component of the aromatase enzyme complex responsible for the final step
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.