Y0001511
Glibenclamide for peak identification
European Pharmacopoeia (EP) Reference Standard
동의어(들):
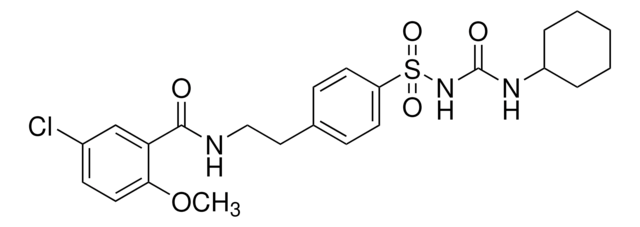
Glybenclamide, 5-Chloro-N-[4-(cyclohexylureidosulfonyl)phenethyl]-2-methoxybenzamide, Glyburide, N-p-[2-(5-Chloro-2-methoxybenzamido)ethyl]benzenesulfonyl-N′-cyclohexylurea
About This Item
추천 제품
Grade
pharmaceutical primary standard
API family
glibenclamide
제조업체/상표
EDQM
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-8°C
SMILES string
COc1ccc(Cl)cc1C(=O)NCCc2ccc(cc2)S(=O)(=O)NC(=O)NC3CCCCC3
InChI
1S/C23H28ClN3O5S/c1-32-21-12-9-17(24)15-20(21)22(28)25-14-13-16-7-10-19(11-8-16)33(30,31)27-23(29)26-18-5-3-2-4-6-18/h7-12,15,18H,2-6,13-14H2,1H3,(H,25,28)(H2,26,27,29)
InChI key
ZNNLBTZKUZBEKO-UHFFFAOYSA-N
유전자 정보
human ... ABCC8(6833) , KCNJ11(3767)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
애플리케이션
포장
기타 정보
관련 제품
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Aquatic Chronic 4
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
이미 열람한 고객
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.