Y0001112
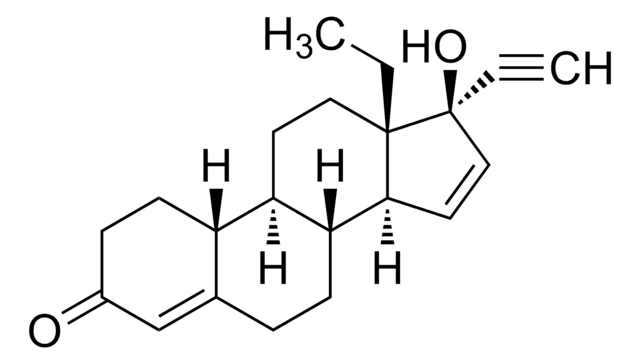
Gestodene
European Pharmacopoeia (EP) Reference Standard
동의어(들):
(17α)-13-Ethyl-17-hydroxy-18,19-Dinorpregna-4,15-dien-20-yn-3-one, Gestinol, SHB 331, WL 70
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
실험식(Hill 표기법):
C21H26O2
CAS Number:
Molecular Weight:
310.43
MDL number:
UNSPSC 코드:
41116107
PubChem Substance ID:
NACRES:
NA.24
추천 제품
Grade
pharmaceutical primary standard
API family
gestodene
제조업체/상표
EDQM
응용 분야
pharmaceutical (small molecule)
형식
neat
SMILES string
CC[C@]12CC[C@H]3[C@@H](CCC4=CC(=O)CC[C@H]34)[C@@H]1C=C[C@@]2(O)C#C
InChI
1S/C21H26O2/c1-3-20-11-9-17-16-8-6-15(22)13-14(16)5-7-18(17)19(20)10-12-21(20,23)4-2/h2,10,12-13,16-19,23H,3,5-9,11H2,1H3/t16-,17+,18+,19-,20-,21-/m0/s1
InChI key
SIGSPDASOTUPFS-XUDSTZEESA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Gestodene EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
생화학적/생리학적 작용
Gestodene is a synthetic progestin used as a contraceptive. Gestodene displays a high binding affinity to the progesterone receptor, and also binds strongly to adrogen and glucocorticoid receptors.
포장
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
기타 정보
Sales restrictions may apply.
신호어
Warning
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Repr. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
가장 최신 버전 중 하나를 선택하세요:
Ladakan Jaithitivit et al.
Journal of the Medical Association of Thailand = Chotmaihet thangphaet, 95(5), 630-635 (2012-09-22)
To determine cycle control, safety, and acceptability of a 24-day oral contraceptive regimen containing 15 micrograms of ethinylestradiol and 60 micrograms of gestodene. This was an open-label, non-comparative study. Healthy women 18 to 35 years old who attended the Family
Romana Dmitrovic et al.
Obstetrics and gynecology, 119(6), 1143-1150 (2012-05-24)
To estimate whether continuous oral contraceptive pills (OCPs) will result in more pain relief in primary dysmenorrhea patients than cyclic OCPs, which induce withdrawal bleeding with associated pain and symptoms. We conducted a double-blind, randomized, controlled trial comparing continuous to
Sopon Cheewadhanaraks et al.
Gynecologic and obstetric investigation, 74(2), 151-156 (2012-06-23)
To evaluate the efficacy and tolerability of postoperative depot medroxyprogesterone acetate (DMPA) versus postoperative continuous oral contraceptive (OC) pills in the treatment of endometriosis-associated pain. After a conservative surgery, 84 patients with symptomatic endometriosis were randomized to receive either intramuscular
Øjvind Lidegaard et al.
BMJ (Clinical research ed.), 343, d6423-d6423 (2011-10-27)
To assess the risk of venous thromboembolism from use of combined oral contraceptives according to progestogen type and oestrogen dose. National historical registry based cohort study. Four registries in Denmark. Non-pregnant Danish women aged 15-49 with no history of thrombotic
Hormone-based contraceptive therapy and risk of venous thromboembolism in young women.
Helen Roberts
Clinical advances in hematology & oncology : H&O, 8(5), 307-309 (2010-06-17)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.