추천 제품
Grade
pharmaceutical primary standard
API family
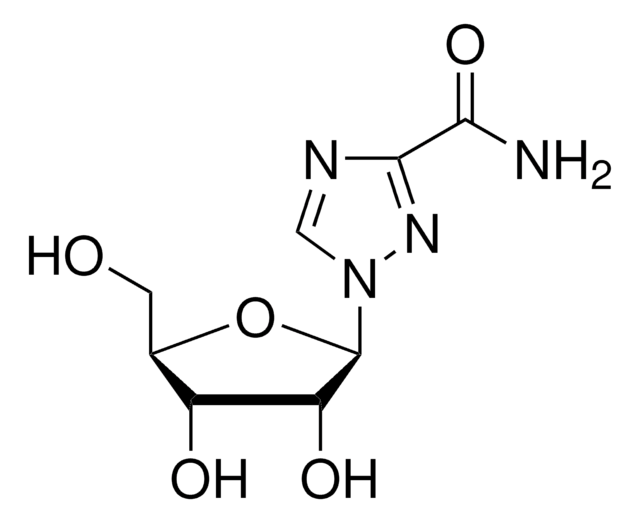
ribavirin
제조업체/상표
EDQM
응용 분야
pharmaceutical (small molecule)
형식
neat
저장 온도
2-8°C
SMILES string
NC(=O)c1ncn(n1)[C@@H]2O[C@H](CO)[C@@H](O)[C@H]2O
InChI
1S/C8H12N4O5/c9-6(16)7-10-2-12(11-7)8-5(15)4(14)3(1-13)17-8/h2-5,8,13-15H,1H2,(H2,9,16)/t3-,4-,5-,8-/m1/s1
InChI key
IWUCXVSUMQZMFG-AFCXAGJDSA-N
유전자 정보
human ... IMPDH1(3614)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Analog of the imidazole nucleotide intermediates in purine nucleotide biosynthesis.
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the Issuing Pharmacopoeia. For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Ribavirin EP Reference standard, intended for use in laboratory tests only as specifically prescribed in the European Pharmacopoeia.
생화학적/생리학적 작용
Antiviral agent used against a wide variety of human viral infections, in particular, chronic hepatitis C, HIV, and adenovirus. Its metabolite, ribavirin 5′-phosphate, is an inhibitor of inosine monophosphate (IMP) dehydrogenase, but many other mechanisms of action are also supported with experimental evidence.
포장
The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity, please visit the EDQM reference substance catalogue.
기타 정보
Sales restrictions may apply.
관련 제품
제품 번호
설명
가격
신호어
Danger
유해 및 위험 성명서
예방조치 성명서
Hazard Classifications
Muta. 2 - Repr. 1B
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
Fred Poordad et al.
The New England journal of medicine, 370(21), 1973-1982 (2014-04-15)
Interferon-containing regimens for the treatment of hepatitis C virus (HCV) infection are associated with increased toxic effects in patients who also have cirrhosis. We evaluated the interferon-free combination of the protease inhibitor ABT-450 with ritonavir (ABT-450/r), the NS5A inhibitor ombitasvir
Eric Druyts et al.
Clinical infectious diseases : an official publication of the Infectious Diseases Society of America, 56(7), 961-967 (2012-12-18)
A systematic review and meta-analysis were conducted to examine the efficacy and safety of pegylated interferon (peg-IFN) alfa-2a and peg-IFN alfa-2b plus ribavirin (RBV) in children and adolescents with chronic hepatitis C virus (HCV). Medline, Embase, and Cochrane Central Register
Michael Manns et al.
Lancet (London, England), 384(9954), 1597-1605 (2014-08-01)
An unmet need exists for interferon-free and ribavirin-free treatments for chronic hepatitis C virus (HCV) infection. In this study, we assessed all-oral therapy with daclatasvir (NS5A replication complex inhibitor) plus asunaprevir (NS3 protease inhibitor) in patients with genotype 1b infection
Chen-Hua Liu et al.
Annals of internal medicine, 159(11), 729-738 (2013-12-04)
Data are limited on the efficacy and safety of pegylated interferon plus ribavirin for patients with hepatitis C virus genotype 1 (HCV-1) receiving hemodialysis. To compare the efficacy and safety of combination therapy with pegylated interferon plus low-dose ribavirin and
Tung-Hung Su et al.
Proceedings of the National Academy of Sciences of the United States of America, 110(19), 7844-7849 (2013-04-25)
MicroRNA-122 (miR-122) facilitates hepatitis C virus replication in vitro. Serum miR-122 has been implicated as a biomarker for various liver diseases; however, its role in chronic hepatitis C remains unclear. To address this issue, 126 patients with chronic hepatitis C
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.