T35920
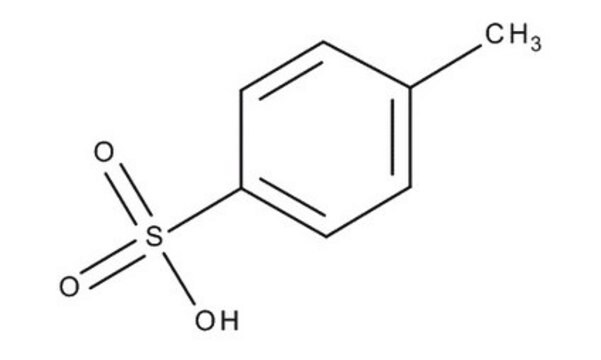
p-Toluenesulfonic acid monohydrate
ReagentPlus®, ≥98%
동의어(들):
4-Methylbenzenesulfonic acid monohydrate, 4-Toluenesulfonic acid monohydrate, PTSA monohydrate, TsOH monohydrate
로그인조직 및 계약 가격 보기
모든 사진(4)
About This Item
Linear Formula:
CH3C6H4SO3H · H2O
CAS Number:
Molecular Weight:
190.22
Beilstein:
3568023
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.21
추천 제품
애플리케이션
p-toluenesulfonic acid monohydrate may be used as a better alternative to Friedel-Crafts catalysts for the alkylation of the aromatic nucleus with activated alkyl halides, alkenes, or tosylates under mild conditions.
법적 정보
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Eye Dam. 1 - Met. Corr. 1 - Skin Corr. 1C - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 1
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Detailed characterization of p-toluenesulfonic acid monohydrate as a convenient, recoverable, safe, and selective catalyst for alkylation of the aromatic nucleus.
Mahindaratne MPD and Wimalasena K.
The Journal of Organic Chemistry, 63(9), 2858-2866 (1998)
Jhonny Azuaje et al.
ACS combinatorial science, 13(1), 89-95 (2011-01-21)
We document here the use of polymer-supported p-toluenesulfonic acid as a highly effective, robust, economical and eco-friendly isocyanide scavenger. The herein described strategy circumvent the intense and repulsive odor of volatile isocyanides, enabling simplified and odorless workup and purifications. The
Aditya Kulkarni et al.
Organic letters, 13(19), 5124-5127 (2011-09-10)
An environmentally benign procedure for the hydrogenation of unprotected indoles is described. The hydrogenation reaction is catalyzed by Pt/C and activated by p-toluenesulfonic acid in water as a solvent. The efficacy of the method is illustrated by the hydrogenation of
Koneni V Sashidhara et al.
Bioorganic & medicinal chemistry letters, 22(12), 3926-3930 (2012-05-23)
First synthesis of novel coumarin-trioxane hybrids is reported. The synthesis was achieved via condensation of β-hydroxyhydroperoxides with coumarinic-aldehydes in presence of p-toluenesulfonic acid in good yields and the novel hybrids were evaluated for their antimalarial activity both in vitro and
Daniel C Yee et al.
The FEBS journal, 280(22), 5780-5800 (2013-08-29)
Visual rhodopsins are recognized members of the large and diverse family of G protein-coupled receptors (GPCRs), but their evolutionary origin and relationships to other proteins are not known. In a previous paper [Shlykov MA, Zheng WH, Chen JS & Saier
프로토콜
Separation of p-Toluenesulfonic acid (PTSA); Pyrimethamine
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.