추천 제품
Quality Level
제품 라인
ReagentPlus®
분석
≥99%
refractive index
n20/D 1.587 (lit.)
bp
188 °C (lit.)
mp
−15 °C (lit.)
density
1.057 g/mL at 20 °C (lit.)
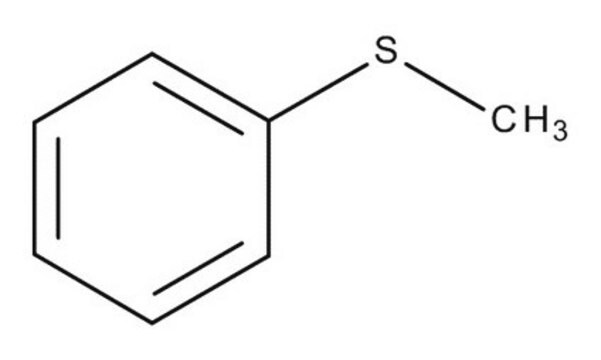
작용기
thioether
SMILES string
CSc1ccccc1
InChI
1S/C7H8S/c1-8-7-5-3-2-4-6-7/h2-6H,1H3
InChI key
HNKJADCVZUBCPG-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
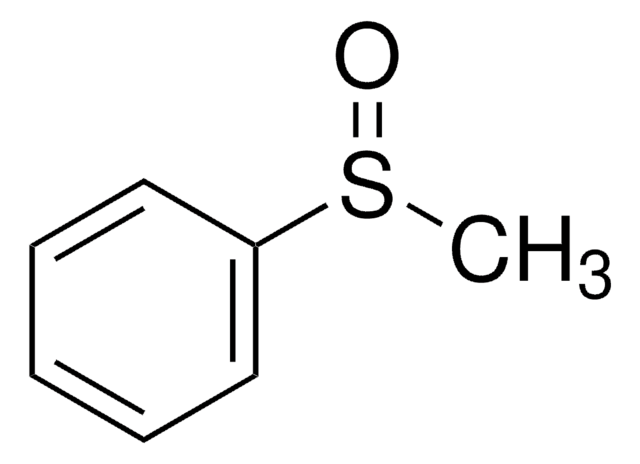
Thioanisole may be used in the synthesis of methyl phenyl sulfoxide via oxidation.
법적 정보
ReagentPlus is a registered trademark of Merck KGaA, Darmstadt, Germany
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Aquatic Chronic 2 - Eye Irrit. 2 - Skin Irrit. 2 - Skin Sens. 1B
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point (°F)
163.4 °F - closed cup
Flash Point (°C)
73 °C - closed cup
개인 보호 장비
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
이미 열람한 고객
Methyl phenyl sulfoxide.
Johnson CR & Keiser JE.
Organic Syntheses, 78-78 (1966)
Mild and selective oxidation of sulfur compounds in trifluoroethanol: diphenyl disulfide and methyl phenyl sulfoxide.
Ravikumar KS, et al.
Organic Syntheses, 184-189 (2003)
Rémy Ricoux et al.
Organic & biomolecular chemistry, 7(16), 3208-3211 (2009-07-31)
Two new artificial hemoproteins or "hemozymes", obtained by non covalent insertion of Fe(III)-meso-tetra-p-carboxy- and -p-sulfonato-phenylporphyrin into xylanase A from Streptomyces lividans, were characterized by UV-visible spectroscopy and molecular modeling studies, and were found to catalyze the chemo- and stereoselective oxidation
Jiyun Park et al.
Journal of the American Chemical Society, 133(14), 5236-5239 (2011-03-18)
The mechanism of sulfoxidation of thioaniosoles by a non-heme iron(IV)-oxo complex is switched from direct oxygen transfer to metal ion-coupled electron transfer by the presence of Sc(3+). The switch in the sulfoxidation mechanism is dependent on the one-electron oxidation potentials
Jun-Long Zhang et al.
Chemical communications (Cambridge, England), (14)(14), 1665-1667 (2008-03-28)
We demonstrate that incorporation of MnSalen into a protein scaffold enhances the chemoselectivity in sulfoxidation of thioanisole and find that both the polarity and hydrogen bonding of the protein scaffold play an important role in tuning the chemoselectivity.
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.