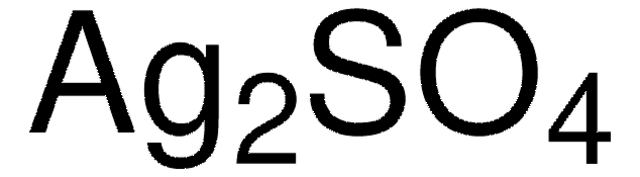추천 제품
제조업체/상표
BP
drug control
estupefaciente (Spain); Decreto Lei 15/93: Tabela IA (Portugal)
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
This product is provided as delivered and specified by the issuing Pharmacopoeia. All information provided in support of this product, including SDS and any product information leaflets have been developed and issued under the Authority of the issuing Pharmacopoeia.
For further information and support please go to the website of the issuing Pharmacopoeia.
For further information and support please go to the website of the issuing Pharmacopoeia.
애플리케이션
Codeine phosphate BP Reference standard, intended for use in laboratory tests only as specifically prescribed in the British Pharmacopoeia.
Also used in monographs such as:Paracetamol, Codeine Phosphate and Caffeine Capsules Co-dydramol Tablets Co-codamol Capsules Co-codamol Tablets Co-codamol Effervescent Tablets Paracetamol, Codeine Phosphate and Caffeine Tablets Morphine Tablets Morphine Prolonged-release Tablets Dihydrocodeine Prolonged-release Tablets Morphine Prolonged-release Capsules Morphine Granules for Oral Suspension Dihydrocodeine Oral Solution Co-codaprin Tablets Dihydrocodeine Injection Morphine Oral Solution Dihydrocodeine Tablets Morphine Suppositories Morphine Capsules Morphine Sulfate Injection Codeine Phosphate Oral Solution
Also used in monographs such as:
포장
Unit quantity: 200 mg. Subject to change. The product is delivered as supplied by the issuing Pharmacopoeia. For the current unit quantity please visit British Pharmacopoeia
기타 정보
Sales restrictions may apply.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 3 Oral
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
가장 최신 버전 중 하나를 선택하세요:
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.