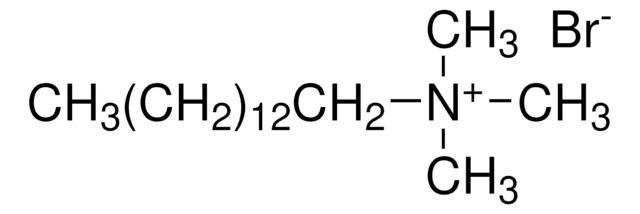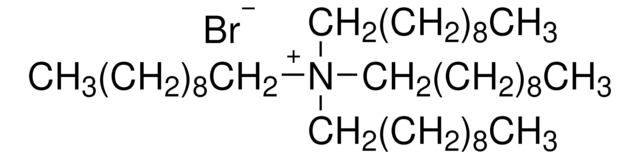87210
Myristyltrimethylammonium bromide
98% (AT)
동의어(들):
Tetradecyltrimethylammonium bromide, Trimethyl(tetradecyl)ammonium bromide
로그인조직 및 계약 가격 보기
모든 사진(1)
About This Item
Linear Formula:
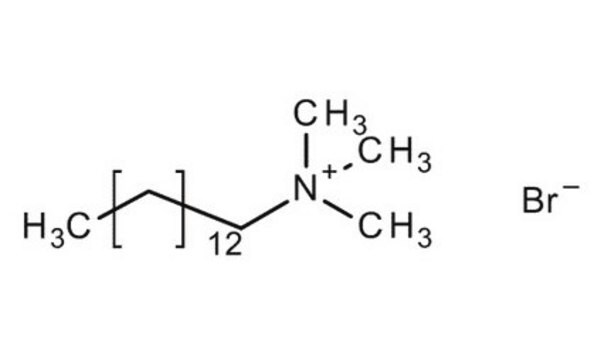
CH3(CH2)13N(Br)(CH3)3
CAS Number:
Molecular Weight:
336.39
Beilstein:
3633227
EC Number:
MDL number:
UNSPSC 코드:
12352116
PubChem Substance ID:
NACRES:
NA.22
추천 제품
설명
cationic
Quality Level
분석
≥97.5% (AT)
98% (AT)
양식
crystals
분자량
micellar avg mol wt 27,000
응집 번호
80
CMC
4-5 mM (20-25°C)
mp
245-250 °C (lit.)
solubility
H2O: 0.1 g/mL, clear
SMILES string
[Br-].CCCCCCCCCCCCCC[N+](C)(C)C
InChI
1S/C17H38N.BrH/c1-5-6-7-8-9-10-11-12-13-14-15-16-17-18(2,3)4;/h5-17H2,1-4H3;1H/q+1;/p-1
InChI key
CXRFDZFCGOPDTD-UHFFFAOYSA-M
유사한 제품을 찾으십니까? 방문 제품 비교 안내
일반 설명
Myristyltrimethylammonium bromide (MTAB) is a quaternary ammonium compound that consists of a long-chain alkyl group and a positively charged quaternary ammonium group. It is commonly used as a cationic surfactant and as a phase transfer catalyst in various chemical reactions.
애플리케이션
Myristyltrimethylammonium bromide can be used as a cationic surfactant to:
- Enhance the biomass harvesting and pigments extraction from the cyanobacterium Synechocystis sp. PCC 6803.
- Facilitate the formation of mixed micelles with amitriptyline hydrochloride.
신호어
Danger
유해 및 위험 성명서
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1 - Eye Dam. 1 - Skin Irrit. 2 - STOT RE 2 Oral - STOT SE 3
표적 기관
Gastrointestinal tract, Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
이미 열람한 고객
Aline Delbos et al.
Physical review. E, Statistical, nonlinear, and soft matter physics, 84(1 Pt 1), 011404-011404 (2011-08-27)
We investigate experimentally the behavior of liquid foams pumped at a given flow rate through a single pore, in the situation where the pore diameter is smaller than the bubble diameter. Results reveal that foam invasion can be observed only
Irma Orentaitė et al.
Electrophoresis, 32(5), 604-613 (2011-02-04)
For tetradecyltrimethylammonium bromide in boric acid/borate or acetic acid/acetate buffer and NaCl or CaCl₂ as the added salt, it is investigated whether the retention behaviour of weak acids in MEKC with cationic surfactant can be modelled by assuming for the
Yuri Park et al.
Journal of colloid and interface science, 360(2), 440-456 (2011-05-24)
Organoclays were synthesised through ion exchange of a single surfactant for sodium ions, and characterised by a range of method including X-ray diffraction (XRD), BET, X-ray photoelectron spectroscopy (XPS), thermogravimetric analysis (TGA), Fourier transform infrared spectroscopy (FT-IR), and transmission electron
Sanket Joshi et al.
Molecular cancer therapeutics, 9(7), 1995-2006 (2010-06-24)
The endocytic protein dynamin II (dynII) participates in cell cycle progression and has roles in centrosome cohesion and cytokinesis. We have described a series of small-molecule inhibitors of dynamin [myristyl trimethyl ammonium bromides (MiTMAB)] that competitively interfere with the ability
Dulan B Gunasekara et al.
Electrophoresis, 32(8), 832-837 (2011-03-26)
The combination of microchip electrophoresis with amperometric detection leads to a number of analytical challenges that are associated with isolating the detector from the high voltages used for the separation. While methods such as end-channel alignment and the use of
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.