모든 사진(1)
About This Item
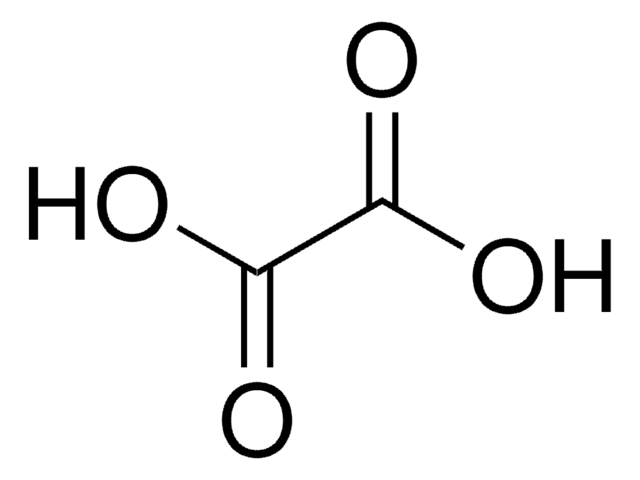
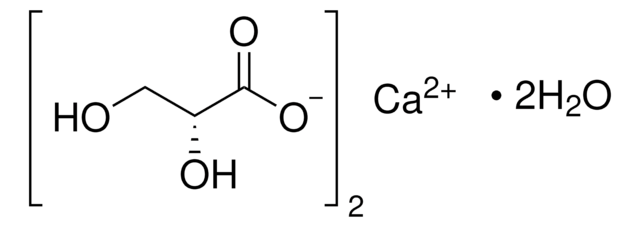
Linear Formula:
HOCH(COOH)2
CAS Number:
Molecular Weight:
120.06
Beilstein:
1209791
EC Number:
MDL number:
UNSPSC 코드:
12352100
PubChem Substance ID:
NACRES:
NA.22
추천 제품
분석
≥97.0%
양식
powder
mp
158-160 °C (dec.) (lit.)
작용기
carboxylic acid
hydroxyl
저장 온도
2-8°C
SMILES string
OC(C(O)=O)C(O)=O
InChI
1S/C3H4O5/c4-1(2(5)6)3(7)8/h1,4H,(H,5,6)(H,7,8)
InChI key
ROBFUDYVXSDBQM-UHFFFAOYSA-N
유사한 제품을 찾으십니까? 방문 제품 비교 안내
애플리케이션
- Polymer synthesis for enhanced thermal conductivity: Tartronic acid is used to exploit enzyme reactions in polymer synthesis, significantly increasing the thermal conductivity of materials, which is pivotal in manufacturing and material science applications (Nan et al., 2023).
- Advances in green chemical treatments: This acid plays a role in the electro-oxidation pathways for treating glycerol waste, contributing to sustainable chemical processes and green chemistry applications, which are essential for reducing environmental impact (Cheng et al., 2021).
- Development in biodiesel by-products treatment: Tartronic acid is also involved in kinetic studies for the electrochemical conversion of glycerol, a by-product of biodiesel production, highlighting its role in renewable energy and waste valorization (Pérès et al., 2020).
- Base-free oxidation reactions: It aids in the development of base-free conditions for glycerol to glyceraldehyde oxidation reactions over platinum-based catalysts, offering advancements in catalysis and organic synthesis processes (Capron et al., 2019).
신호어
Warning
유해 및 위험 성명서
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
표적 기관
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point (°F)
Not applicable
Flash Point (°C)
Not applicable
개인 보호 장비
dust mask type N95 (US), Eyeshields, Gloves
이미 열람한 고객
G DuVal et al.
Biochemistry, 24(8), 2067-2072 (1985-04-09)
Some kinetic characteristics of immobilized native mitochondrial malate dehydrogenase dimers and immobilized protomers, prepared by direct immobilization under conditions yielding complete dissociation without substantial unfolding, were compared to those of native soluble enzyme. Enzyme was covalently immobilized to derivatized porous
J K Hiltunen et al.
Biochimica et biophysica acta, 678(1), 115-121 (1981-11-18)
The mechanism of depletion of tricarboxylic acid cycle intermediates by isolated rat heart mitochondria was studied using hydroxymalonate (an inhibitor of malic enzymes) and mercaptopicolinate (an inhibitor of phosphoenolpyruvate carboxykinase) as tools. Hydroxymalonate inhibited the respiration rate of isolated mitochondria
Christopher D Evans et al.
The Journal of chemical physics, 152(13), 134705-134705 (2020-04-10)
The oxidation of glycerol under alkaline conditions in the presence of a heterogeneous catalyst can be tailored to the formation of lactic acid, an important commodity chemical. Despite recent advances in this area, the mechanism for its formation is still
A dynamic kinetic asymmetric transformation in the alpha-hydroxylation of racemic malonates and its application to biologically active molecules.
Dhande Sudhakar Reddy et al.
Angewandte Chemie (International ed. in English), 48(4), 803-806 (2008-12-23)
Formation of 2-keto-D-gluconic acid, 5-keto-D-gluconic acid, and tartronic acid by Acetobacter species.
D KULKA et al.
Nature, 167(4257), 905-906 (1951-06-02)
자사의 과학자팀은 생명 과학, 재료 과학, 화학 합성, 크로마토그래피, 분석 및 기타 많은 영역을 포함한 모든 과학 분야에 경험이 있습니다..
고객지원팀으로 연락바랍니다.